The intracellular environment: not so muddy waters Understand article
Giuseppe Zaccai from the Institut Laue-Langevin (ILL) in Grenoble, France, describes how he and his co-workers have uncovered a way to explore water dynamics in the cell interior using neutron scattering and isotope labelling.

Image courtesy of EdwardShtern
/ iStockphoto
Compared to other liquids, water has extraordinary properties. As water is essential for all living organisms, its properties also play a truly vital role at the level of molecular biology – a discipline which seeks to understand life processes at the levels of atoms, molecules and their interactions.
The hydrophobic effect is one such property. It describes the observation that in a liquid solution, water and oil do not mix. The reason is that water molecules can form hydrogen bonds with each other and other molecules (which are called hydrophilic), but not with oil-like molecules (which are called hydrophobic) (for more on this topic, see Cicognani, 2006). This has fundamental consequences in molecular biology. The hydrophobic effect leads to the spontaneous organisation of lipid molecules to form the membranes that surround cells. It also contributes to the formation of three-dimensional structures in proteins, RNA and DNA, favouring their folding in such a way as to hide the hydrophobic parts of their structures from contact with water and to expose the hydrophilic parts.

Zaccai at ILL
Image courtesy of Giuseppe Zaccai
The hydrophobic effect, as it is understood, depends critically on the special dynamic molecular properties of liquid water. The implication of this effect in membrane formation and macromolecular folding was deduced from test-tube experiments on solutions in which water is clearly in the liquid state. There have been suggestions, however, that water in cells is not in its normal liquid state but is somehow ‘tamed’ and cannot move about as freely inside the viscous intracellular environment, a thick soup of proteins and other molecules.
It was therefore very important to measure the dynamic state of water directly in living cells. This was not an easy task, but the special properties of the neutron helped scientists from my research group at the ILLw1, as well as researchers at the Institut de Biologie Structurale CEA-CNRS-UJFw2 in Grenoble, France, to tackle it successfully.

marismortui cells. Note the red
colour of the halophilic organisms,
which in natural environments
colours salt lakes and salt ponds.
It is due to carotenoids in the cell
membranes, which enter the food
chain and are also responsible,
for example, for the colour of pink
flamingos
Image courtesy of Giuseppe Zaccai
The first experiments on water dynamics in living cells were performed at ILL on cells from organisms that live in the extremely salty conditions of the Dead Sea (Tehei et al., 2007). Salt is used as a preservative because at high concentration it usually kills micro-organisms. The Dead Sea halophilic (salt-loving) organisms evolved to cope with the very high salt concentration by having macromolecules with markedly increased hydrophilic surfaces. These surfaces affect water dynamics inside the cell, leading to the observation of a major ‘slow water’ component in the Dead Sea cells.
Clearly, if this were true for all organisms, it would lead to a complete reassessment not only of the hydrophobic effect, but also of the role of water in biology in general. It was therefore essential to test whether this behaviour was special to the halophilic organisms or could be generalised (Jasnin et al., 2008).
At ILL, scientists use neutron beams to investigate a variety of solid and liquid materials. In neutron spectrometry experiments to measure dynamics (how atoms move in a substance), the neutrons in the beam collide with the atoms to be studied, like billiard balls bouncing off each other. Neutrons and atoms exchange energy and momentum – the neutrons are scattered. Thus, measuring how these values change for the neutrons after the collision gives us an indication of the energy and momentum of the atoms they encountered, and therefore of how these atoms move.
But how can we distinguish between the motions of different atoms in a complex sample, such as a cell that contains not only water but also many other molecules whose atoms move in different ways? Neutrons are scattered with different power by different atoms. To study complex systems, scientists use a trick to reduce the scattering power of everything they do not want to measure. Hydrogen scatters neutrons much more strongly than all other atom types (about 10-100 times, depending on which atom type you compare it with). In contrast, deuterium, a heavy isotope of hydrogen (its nucleus contains one neutron in addition to one proton), scatters neutrons about 40 times more weakly than hydrogen. Exploiting this property, scientists replace hydrogen with deuterium in the components of a complex system they are not interested in and render them practically ‘invisible’. The contributions to the scattering signal by the molecules that contain deuterium are negligible; we ‘see’ only the motions of the molecules that contain hydrogen.

sample on the cryostat rod
of the IN6 spectrometer.
The cryostat controls the
sample temperature during
data collection. The sample
is in the flat aluminium box
at the end of the rod.
The discs on the rod are
baffles to help maintain
constant temperature at the
bottom of the cryostat
Image courtesy of Giuseppe
Zaccai
Marion Jasnin and her co-workers used this trick to analyse water dynamics in vivo in the cytoplasm of Escherichia coli bacteria, taking advantage of the neutron sources at ILL and ISISw3, UK. Studying physics with biological samples is always a difficult task, and human cells are very delicate and complicated to work with. E. coli were a good alternative as they are easier to handle, yet live in the human gut under similar physiological conditions of temperature and salinity as our own cells; and remember, the adaptation of the cytoplasm to high salinity was thought to be the cause of the ‘slowed down’ water in the halophiles.
To replace the hydrogen atoms in the proteins and other cellular macromolecules by deuterium, E. coli cells were grown on deuterated nutrients and deuterated (heavy) water. For the measurements, they were then centrifuged gently and the heavy water was replaced with normal (hydrogen-containing) water, diluting out the deuterium-containing intracellular water but not the deuterium in the macromolecules. In such a sample, after diluting out, the neutron scattering signal comes mainly from the intracellular water. The pellet of living cells was placed in an aluminium sample holder. Aluminium, like all metals, is transparent to neutrons – though obviously not to light or X-rays.
Neutron energy and momentum are determined before and after scattering by measuring their wavelength (in the Ångström range). The two main methods used to do this (depending on the spectrometer) are by ‘time of flight’, in which the neutron velocity (inversely proportional to wavelength, velocity is in the km/s range for Ångström wavelengths) is measured over a determined path; and by diffraction of crystals (according to Bragg’s law, only a certain wavelength is diffracted for a given crystal periodicity and angular setting – read more about this law in Hughes, 2007 and Cornuéjols, 2009). Find out more about these methods onlinew4.
Heat is motion: the speed at which atoms in a material move depends on the temperature. However, atoms in one material can also move at different speeds at the same temperature, depending on how they are bound to other atoms around them: water molecules are known to be slowed down by direct contact with macromolecules such as proteins or DNA. The question the scientists asked was: do cellular water molecules that are not in direct contact with macromolecules move as they would normally in liquid water, or are they, too, significantly slowed down?
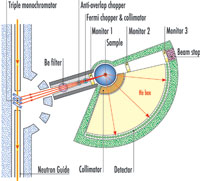
scattering spectrometer at ILL.
Click to enlarge image
Image courtesy of ILL
Each neutron spectrometer is specialised for measuring atomic motions occurring within a given length scale–time scale window. Basically, there are three types: those measuring in the range of about 1 Ångström amplitude occurring in about 1 picosecond (10-12 s), which corresponds to the thermal motion of hydrogen atoms in liquid water at room temperature (note that this corresponds to speeds of about 100 m/s); those measuring in the range of 1-10 Ångström amplitudes in a nanosecond (10-9 s), which would pick up ‘slowed down’ water; and an intermediate type for the range of 1-10 Ångström amplitudes in 100 picoseconds.
By using a picosecond and a nanosecond spectrometer, Marion Jasnin and her co-workers established that water dynamics within a bacterial cell are similar to those in pure water. Water molecules rotate as well as diffuse linearly in the liquid, and a slightly slowed-down rotational diffusion was measured. From the fraction of hydrogen atoms that moved more slowly and the average surface of macromolecules inside an E. coli cell, the scientists calculated that this fraction corresponds to a single layer of water molecules next to the macromolecules that is slowed down, but the rest flows as freely as in liquid water.
What happens inside the cell, then, is similar to what is found around the islands of the Venetian lagoon in Italy. The water close to the macromolecules (islands) is held up, whereas in between – as little as one layer of water molecules from the macromolecules – the water regains its fluidity. This is in contrast to the ‘taming’ hypothesis that claimed that all the water in the cell would be slowed down.
Following up on the E. coli experiments, the group has now also managed to explore water dynamics in human red blood cells at neutron sources in Germany (FRM IIw5) and Switzerland (PSIw6). The same behaviour as in E. coli was confirmed, with liquid water flowing freely beyond the first layer which is in contact with haemoglobin, the main protein contained in these cells (Stadler et al., 2009).
Scientists can heave a sigh of relief – and continue to do their experiments in liquid water solutions, thanks to this confirmation that such experiments are a valid model for what happens in cells.
References
- Cicognani G (2006): Defying the laws of physics? Science in School 1: 19-21.
- Cornuéjols D (2009): Biological crystals: at the interface between physics, chemistry and biology. Science in School 11: 70-76.
- Hughes D (2007) Taking the stress out of engineering. Science in School 5: 61-65.
- Jasnin M, Moulin M, Haertlein M, Zaccai G, Tehei M (2008) Down to atomic-scale intracellular water dynamics. EMBO Reports 9: 543-547. doi:10.1038/embor.2008.50
- Stadler AM, Embs JP, Digel I, Artmann GM, Unruh T, Buldt G, Zaccai G (2008) Cytoplasmic water and hydration layer dynamics in human red blood cells. Journal of the American Chemical Society 130: 16852-16853. doi:10.1021/ja807691j
- Tehei M, Franzetti B, Wood K, Gabel F, Fabiani E, Jasnin M, Zamponi M, Oesterhelt D, Zaccai G, Ginzburg M, Ginzburg BZ (2007) Neutron scattering reveals extremely slow cell water in a Dead Sea organism. Proceedings of the National Academy of Sciences of the United States of America 104: 766-771. doi:10.1073/pnas.0601639104
Web References
- w1 – To learn more about the Institut Laue-Langevin, see: www.ill.eu
- w2 – To find out more about the Institut de Biologie Structurale CEA-CNRS-UJF, see: www.ibs.fr
- w3 – Learn more about ISIS, the pulsed neutron and muon source located at the UK Rutherford Appleton Laboratory near Oxford, here: www.isis.stfc.ac.uk
- w4 – For more information about neutron diffraction, as well as about the time-of-flight and crystal diffraction techniques, see the following direct links to Wikipedia pages:
- http://tinyurl.com/yh436y4 for neutron diffraction
- http://tinyurl.com/ykywxht for inelastic neutron scattering
- http://tinyurl.com/yj4bnrf for time-of-flight
- http://tinyurl.com/yfho9gz for crystal diffraction.
- w5 – Find out more about the German research neutron source FRM II (Forschungs-Neutronenquelle Heinz Maier-Leibnitz) in Munich here: www.frm2.tum.de
- w6 – To learn more about the Paul Scherrer Institute in Villingen, Switzerland, see: www.psi.ch
Resources
Leigh V (2008) Salt of the Earth. Science in School 8: 60-62.
Institutions
Review
Much is made in school science of the scientific process, and yet our students typically do not have much exposure to cutting-edge research in science, or to the style of writing used in academic journals. The reasons for this distance include little apparent relevance to school science, and the often impenetrable style of academic prose.
These criticisims do not apply to Zaccai’s article, which also addresses an important ‘how do we know?’ question. While the behaviour of molecules in vitro may be well studied and understood, it is often a matter of conjecture how much of this replicates behaviour in vivo. The article suggests that water, at least, does not behave differently, and the text is of interest to teachers and older students of biology, physics or chemistry, particularly as there is a cross-disciplinary nature to the reported studies.
Possible comprehension questions include:
- Explain the analogy drawn between the lagoon in Venice and intracellular water.
- What non-SI unit(s) crop(s) up in the article? Express this/these in SI units.
- What does ‘deuteration’ mean?
- Why was E. coli a good experimental subject?
- Rank some of the materials mentioned in order of their ability to scatter neutrons.
- Define ‘hydrophobic’ and ‘hydrophilic’.
- How were the irrelevant molecules rendered ‘invisible’ to the neutron beam?
- What conclusion can be drawn by the work described in the article?
Ian Francis, UK





