Supporting materials
issue13_antioxidants_reaction.pdf
issue13_antioxidants_table1.pdf
issue13_antioxidants_table2.pdf
Download
Download this article as a PDF

We’ve all heard that an antioxidant-rich diet is healthy. Together with his students, Gianluca Farusi compared the antioxidant levels in a range of foods and drinks.
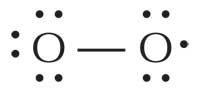
Many health problems, including atherosclerosis, heart attack, Alzheimer’s disease, some tumours and senile cataracts, are associated with very reactive molecules called free radicals. These molecules are produced normally during aerobic respiration and are used by the body, for example to defend it against micro-organisms.
If, however, there is an imbalance between radicals (oxidants) and antioxidants, this can lead to disease.
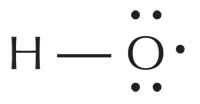
Free radicals are so reactive because they possess one or more unpaired electrons. They are produced and found in many cells and cell organelles; the superoxide anion radical (O2–), for example, is the most common radical in the body, being used by white blood cells to attack viruses and bacteria. By far the most reactive radical, however, is the hydroxyl radical (HO·), found in the peroxisome (where fatty acids are broken down) and the endoplasmic reticulum. External factors influence the production of radicals too; for example, ultraviolet (UV) light shining on our skin causes singlet state oxygen radicals (1ΔO2·) to be formed.
Within the body, free radicals can lead to a variety of problems. In particular, they react with – and damage – lipids, proteins and nucleic acids, including DNA (Arking, 2006). To protect itself from the continuous radical attack, our body has two basic forms of protection: enzymatic and non-enzymatic. The most important enzymes used to defend our bodies from free radical attack are the antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase. The principal non-enzymatic antioxidants are melatonin, α-tocopherol (vitamin E), ascorbic acid (vitamin C) and β-carotene (the vitamin A precursor).
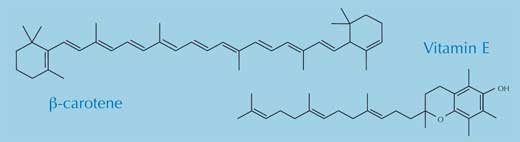
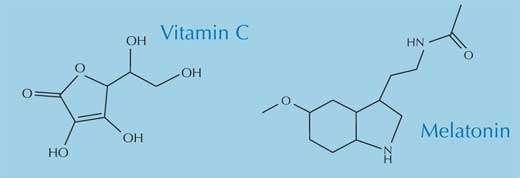
All four non-enzymatic antioxidants are essential in the diet and are found in a range of foods. Cancer in particular is less common among people who eat plenty of fruit and vegetables, and it has been suggested that the health benefits are due to the antioxidants they contain (Polidori et al., 2009; Swirsky Gold et al., 1997), which counteract the damaging effects of free radicals. Currently, there is little evidence that antioxidant supplements (e.g. tablets) have any health benefits.
The experiment below compares the levels of antioxidants in various types of food and drink, i.e. the effectiveness of the different types of food and drink as radical scavengers.
To educate my 17-year-old students about a responsible diet, in the hope of reducing their risk of developing the diseases mentioned above, I designed an activity based on the Briggs-Rauscher reaction: an oscillating reaction in which amber radical and blue non-radical steps alternate.
By adding samples of different types of food and drink to the reaction and measuring the time intervals between colours, the students could compare the effectiveness of the samples as radical scavengers. Of course it is a comparative and not an absolute evaluation. But one thing at a time…
4 M hydrogen peroxide solution: pour 400 ml distilled water into a 1 l flask. Wearing gloves, add 410 ml 30% hydrogen peroxide. Using distilled water, dilute the solution to 1.0 l.
0.20 M potassium iodate and 0.077 M sulphuric acid solution: place 43 g potassium iodate and approximately 800 ml distilled water into a 1 l flask. Add 4.3 ml concentrated sulphuric acid. Warm and stir the mixture until the potassium iodate dissolves. Dilute the solution to 1.0 l with distilled water.
0.15 M malonic acid and 0.20 M manganese sulphate solution: dissolve 16 g malonic acid and 3.4 g manganese (II) sulphate monohydrate in approximately 500 ml distilled water in a 1 l flask. In a 100 ml beaker, heat 50 ml distilled water to boiling. In a 50 ml beaker, mix 0.30 g soluble starch with about 5 ml distilled water and stir the mixture to form a slurry. Pour the slurry into the boiling water and continue heating and stirring the mixture until the starch has dissolved. Pour the starch solution into the solution of malonic acid and manganese (II) sulphate. Dilute the mixture to 1.0 l with distilled water.
Food samples: to prepare the food samples as aqueous solutions or suspensions, put 2.0 g into a 400 ml beaker. Add 100 ml distilled water and stir with a glass rod. Decant, pour a portion into a test tube and centrifuge. For drinks, such as coffee or wine, take 2.0 ml, add 100 ml distilled water and stir.
Into a 100 ml beaker containing a magnetic stirrer, pipette: 10 ml 4 M hydrogen peroxide aqueous solution, 10 ml aqueous solution of 0.20 M potassium iodate and 0.077 M sulphuric acid, and 10 ml aqueous solution of 0.15 M malonic acid and 0.20 M manganese sulphate. Start the magnetic stirring. When the amber solution turns blue for the second time, add 1 ml food solution or suspension. A video of the colour changes is available online.w2
The Briggs-Rauscher reaction is an oscillating reaction – that is, a mixture of chemicals goes through a sequence of colour changes which repeats periodically. The exact mechanism of the reaction is still under investigation, but the nature of the oscillations is sufficiently clear. For the purposes of this article, it is enough to know that as long as the radical process maintains the concentration of the intermediate HIO higher than the concentration of the intermediate I–, the solution remains amber; when the non-radical process takes place, [I–] is greater than [HIO] and the iodide ion combines with I2 to form a blue complex with starch. A more detailed description of the reaction can be downloaded from the Science in School websitew1.

Since we add the food solution or suspension after the second blue phase, when the non-radical phase is ending and the radical phase is about to start, the longer the time interval between the second and the third blue phases, the greater the antioxidant capacity of the food. In other words, the food has reacted with the radicals produced and the reaction takes longer to produce enough radicals to allow the oscillating reaction to continue.
We dedicated much time to choosing the best food or drink concentration to use, since too-dilute solutions lowered the antioxidant capacity and too-concentrated solutions increased the reaction time so much that it was not practical to make a statistically significant number of trials during the course of the lesson.
Both malonic acid and iodine (produced during the reaction) can irritate the skin, eyes and mucous membranes; for this reason the reaction must be carried out in a fume cupboard.
Because 30% hydrogen peroxide is a very strong oxidising agent, eye protection, lab coats and gloves must be worn. Any contact between hydrogen peroxide and combustible materials must be avoided.
Sulphuric acid is a strong dehydrating agent; eye protection, lab coats and gloves must be worn.
To safely dispose of the mixture at the end of the experiment, slowly add sodium thiosulphate (Na2S2O3) to the reaction products, until the excess iodine turns into colourless iodide ions (the reaction is quite exothermic).
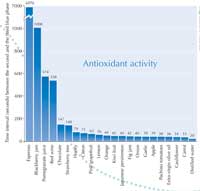
When I performed the activity with my students, we found the greatest antioxidant activity in espresso coffee: 6970 seconds. See graph to the right.
More details of our results can be downloaded from the Science in School website. Table 1 shows the foods we tested, the antioxidant activity (time interval) and the main substance presumed to be responsible for the activity.
What, then, should we conclude from the results? Clearly, a diet consisting purely of espresso may contain high levels of antioxidants but would be far from healthy. When I did this activity with my students, it led to detailed discussion of radical reactions. Below are some questions that could be used to start a discussion.
This is a superb article which underlines the importance of chemistry in the behaviour of biological systems. It is so important to instil an appreciation of the way in which scientific knowledge is multidisciplinary. Teachers and students could use the article for a practical activity in chemistry, biochemistry, food-science or health-science lessons. It could also form the basis of science-fair projects.
If the experimental part were left out, the introduction, discussion and results could be a sound basis for a comprehension exercise. Suitable questions, which could be used to spark off a discussion about food and health, or chemistry in everyday life, could include:
Marie Walsh, Republic of Ireland
issue13_antioxidants_reaction.pdf
issue13_antioxidants_table1.pdf
issue13_antioxidants_table2.pdf
Download this article as a PDF