Supporting materials
Theoretical key points handout
Download
Download this article as a PDF

How to teach radioactive decay and radioisotopes to students who feel that equations are boring? Here are two inexpensive and captivating activities to apply in your classroom!

High-school students might have heard of radiocarbon dating for organic materials, but they might be intimidated by the decay equation as it can feel abstract and dry. While promoting active-participative learning in a 12th grade mathematics-informatics class, I aimed to help students understand the connections between the solutions of the mathematical equations and the behaviours that are observed in the real world. As such, I created a board game with cards to make the connection for given radioisotopes even more obvious. I focused on the probabilistic nature of radioactive decay and on the proportionality between activity and the size of the undecayed population. The students were getting excited not only for working in teams but also because the activity creates just enough of a challenge to spark questions, before imposing answers.
This activity takes between 20 and 35 minutes.
Make sure your students are familiar, at a basic level, with the following concepts, operations, and equations:
If that is not the case, your students may use the theoretical key points handout.
This activity simulates radioactive decay using a competitive, teamwork-based classroom game. We have chosen a number of five teams for a variety of radionuclides, but you may adapt the number of teams depending on your classroom’s configuration. This is a competition between the teams, so you are able to promote fair play and perseverance among students with the help of science. Obviously, while each team benefits from learning about radioactive decay, the team that first and correctly arranges the snapshot cards from the earliest timepoint to the latest wins!


Here is a worked example for the following case:
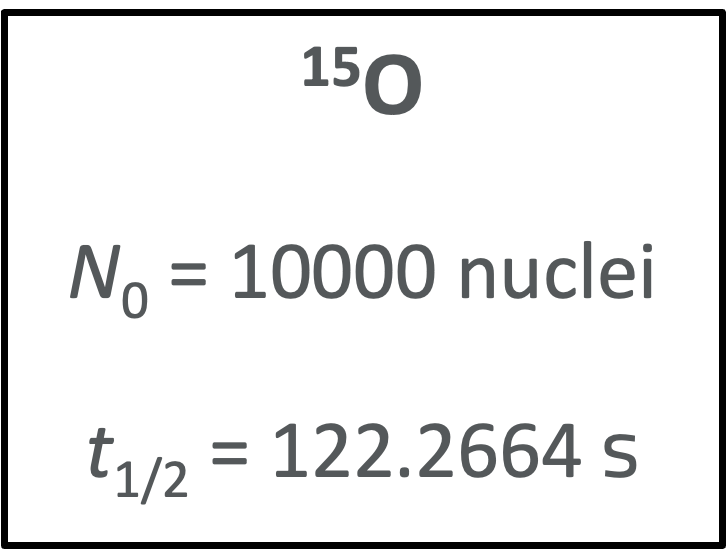
Since we are given the half-life of 15O, we can find the value of the decay constant:
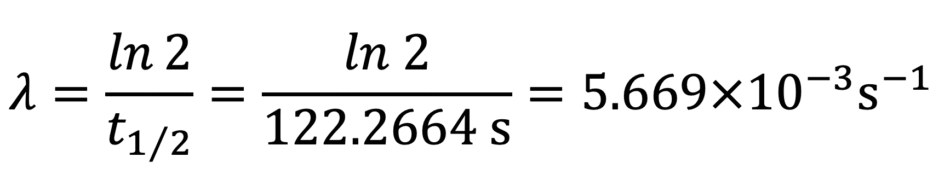
We will write this value on the card with the radionuclide, which will be useful for the next calculations.
According to the law of radioactive decay, t is expressed as a function of ln(N/N0) in the numerator and λ in the denominator (See the theoretical key points handout for a step-by-step guide to calculate t ). Therefore, we can calculate the necessary values for t.
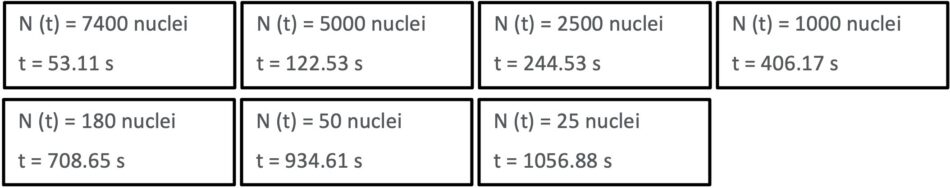
For example, when N (t) = 5 000 nuclei:
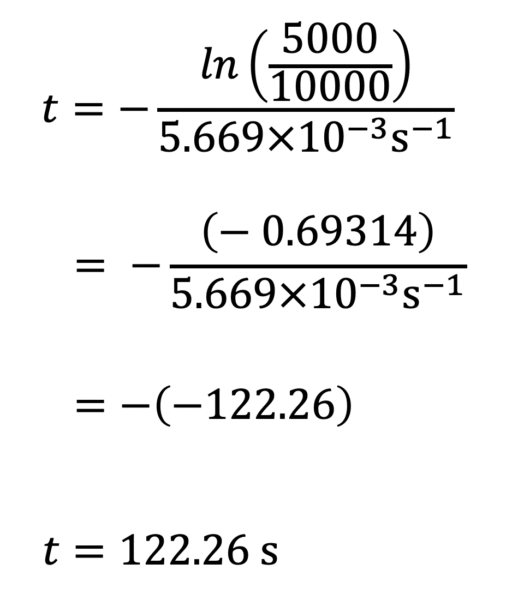
122.26 s corresponds to the half-life (t1/2) of 15O.
The same way we find the other values for t :
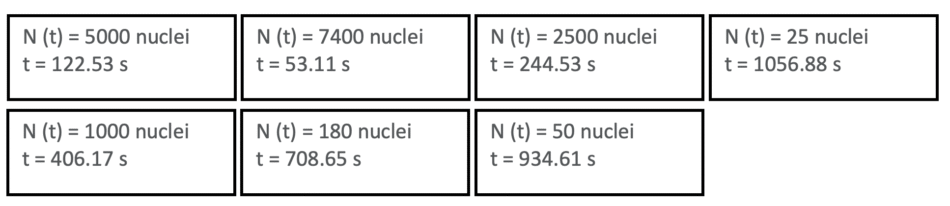
By arranging the ‘snapshot’ cards in order of increasing time values, we can see that the population of undecayed nuclei decreases exponentially over time:
In my experience, students have no significant difficulty in realising the pattern: the numbers don’t decrease linearly, but exponentially. They begin calculating, estimating, arguing. One of the students suggested plotting the data to verify the curve, which could be done using Microsoft Excel or graph paper. Another student even noticed that the activity mirrored the unpredictability of decay in real systems. Most importantly, they internalized the concept without requiring me to provide incremental values for timepoints or to derive the radioactive decay equation.
These questions are meant to evaluate how well the students understand the quantitative aspects of radioactive decay.
This activity takes the form of an inquiry-based group project. Students are invited to work as scientific consultants, using data and background knowledge to analyse how certain properties of radionuclides (half-life, decay constant, energy levels) are applied in real-life scenarios. While this activity typically takes longer than Activity 1 (35–50 min), bear in mind that it may be useful even in classes where mathematics is not taught intensively. As such, it strengthens interdisciplinary understanding by solving realistic problems that require both scientific reasoning and communication.
The students enthusiastically engaged with this real-world challenge. Many were surprised to learn how radioactive decay is applied in medicine and archaeology beyond the usual nuclear physics topics. The team working on radiocarbon dating discussed how the half-life of carbon-14 makes it ideal for ancient samples, not recent ones. And the medical group realized that the short half-life of technetium-99m limits radiation exposure while allowing effective imaging.
Several groups raised insightful questions about public perceptions of radiation, which sparked valuable classroom discussions about radiophobia and responsible science communication. Students appreciated having the opportunity to reason and explain rather than just calculate.
Theoretical key points handout
Download this article as a PDF

Find out how women scientists contributed to knowledge of the chemical elements – and what this tells us about the nature of scientific work, then…

Safety first: nuclear decay and ionizing radiation can be safely studied in the physics classroom using the common baking ingredient potassium carbonate.

You shall not pass: explore the function of deep geological repositories and the key role of bentonite in preventing the leakage of highly radioactive waste.