Supporting materials
Download
Download this article as a PDF

Safety first: nuclear decay and ionizing radiation can be safely studied in the physics classroom using the common baking ingredient potassium carbonate.
Teaching radioactivity in schools often involves challenges related to safety regulations, legal requirements, and cost. Traditional sealed radioactive sources – once a staple in physics classrooms – have become increasingly impractical due to strict regulatory demands, expensive storage and disposal protocols, and declining educational relevance. In Germany, for example, the Strahlenschutzverordnung (Radiation Protection Ordinance)[1] sets stringent activity thresholds, making the use of these sources in schools both legally and logistically difficult.
This article presents an alternative approach: exploring the radioactive properties of potassium-40 using everyday materials like potassium carbonate (K2CO3), commonly known as potash. This compound is inexpensive, widely available, and it contains naturally occurring potassium-40 in quantities far below legal exemption limits.
By using this approach, students can investigate fundamental nuclear physics phenomena – such as beta and gamma radiation, absorption, and half-life – without the need for licensed radioactive sources. At the same time, the activity encourages scientific inquiry; promotes critical thinking; and helps demystify radiation by connecting it to familiar, real-world substances.
You can easily demonstrate the radiation of a food ingredient by holding small unopened paper bags containing 20 g of potassium baking additive directly in front of a sensitive 2-inch Geiger–Müller (G-M) tube, often called a G-M ‘pancake’ detector. Electrons and gamma photons easily pass through the paper envelope and the electrons will produce a signal of approximately 7–10 counts per second (cps), which is significantly higher than the background of approximately 0.5–1.0 cps without any specific source of radiation.
Note: this activity isn’t given in Bq because the detector doesn’t give you the activity of the sample unless you know the energy-dependent efficiency, the distance (geometry) shielding, and self-absorption of the probe.

20 g of potassium carbonate contains approximately 11.3 g potassium, which has a specific activity of approximately 31 Bq/g. The total activity in one small bag is approximately 360 Bq (89.3% beta minus, 10.7% electron capture plus gamma, 0.0% beta plus).[2]

This produces approximately 320 fast electrons per second, with Emax = 1.31 MeV, which easily pass through paper and produce the counts. 34 gamma photons (E = 1.5 MeV) per second will seldomly contribute to your count, because the gamma response of the G-M tube is very low, but the photons are moving quite far. So do the approximately 320 electron antineutrinos per second (Emax = 1.3 MeV), which easily pass through your body, the building, and even through the Earth.
If you use two or three unopened small bags as a stack, the result will be only a very small increase in cps. This is because the beta particles from the second and third layers are absorbed by the sample itself before they can reach the detector. This effect is called self-absorption.
If you use a bigger sample of potassium salt, you will only get beta particles from the outer layer with a thickness of approximately 2.0–5.0 mm.[3]
You can find more information in the radioactivity infosheet in the supporting material, which can also be used as a handout for students during the activities.
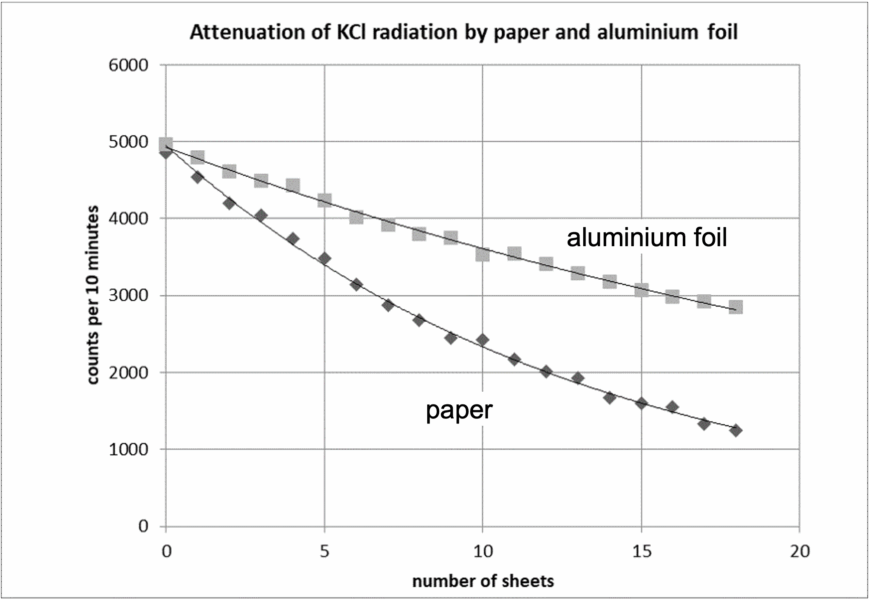
This activity introduces students to the attenuation of beta radiation by different materials. Using potassium carbonate, which is a radiation source, students investigate how the intensity of detected radiation decreases when it passes through shielding materials.
The activity takes 45–60 minutes (which can be split over two lessons).
The observed decrease in count rate reflects the absorption of beta radiation. Paper and thin aluminium block most beta particles.
This activity explores how the intensity of detected radiation depends on the distance from the source by investigating the range of beta particles in air. This experiment supports discussions on radiation type, range, and fundamental laws like geometric dilution.
This activity takes about 45 minutes.
Count rates plotted against distance typically show a sharp initial drop (mainly due to beta absorption in air). At close distances (≤1 cm), beta particles contribute significantly to the count rate. As the distance increases, beta particles are largely absorbed by air.
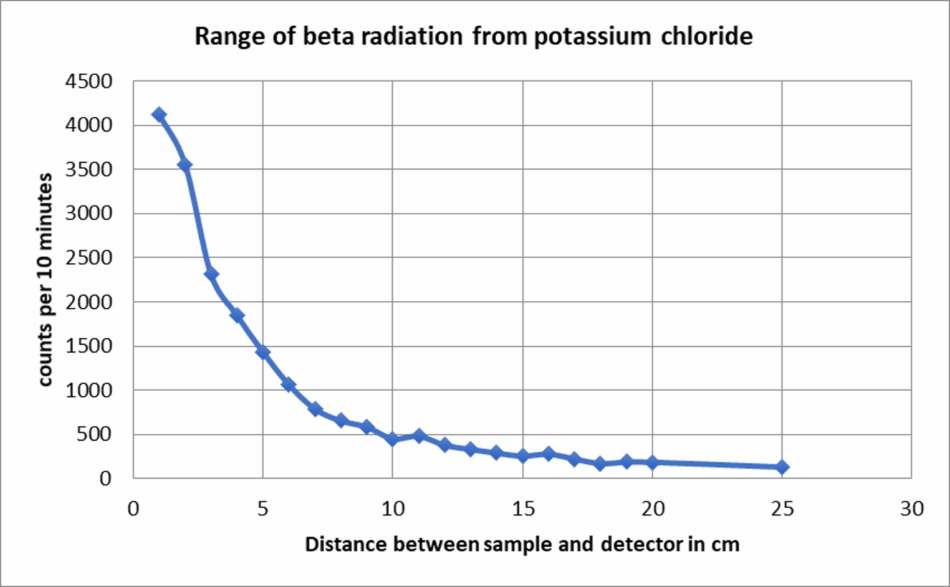
As an extension, students can plot the overlaying theory curve for gamma radiation: I ∝ 1/r 2.
Efficiency and limitations
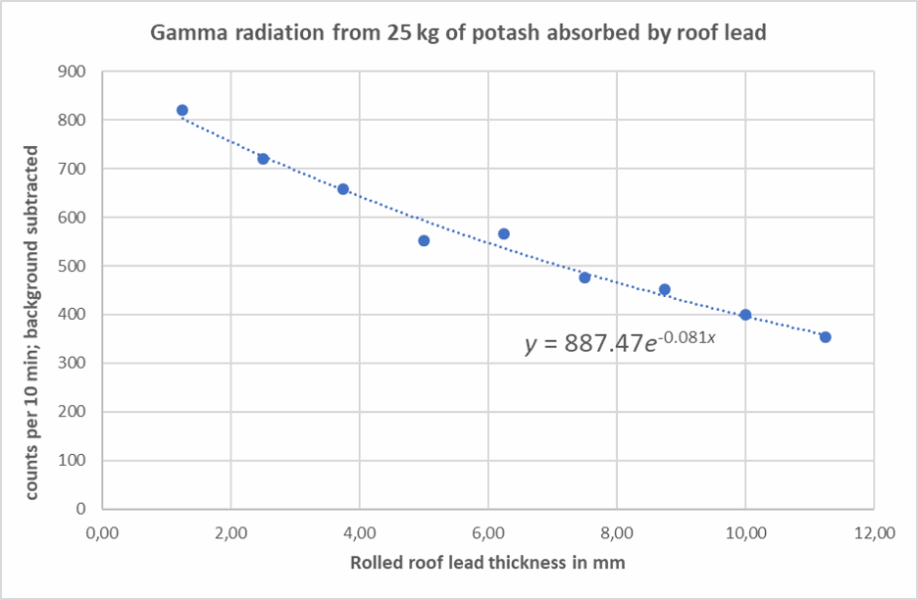
This activity demonstrates the exponential attenuation of gamma radiation as it passes through absorbing materials, such as lead. Students observe how gamma-ray intensity decreases with increasing shielding thickness and evaluate the absorption coefficient for high-energy gamma photons. This is a good opportunity to teach about exponential laws in physics and data analysis skills.
The activity takes about 45 minutes.


Example data:
The logarithmic plot (ln I versus x) should yield a straight line:
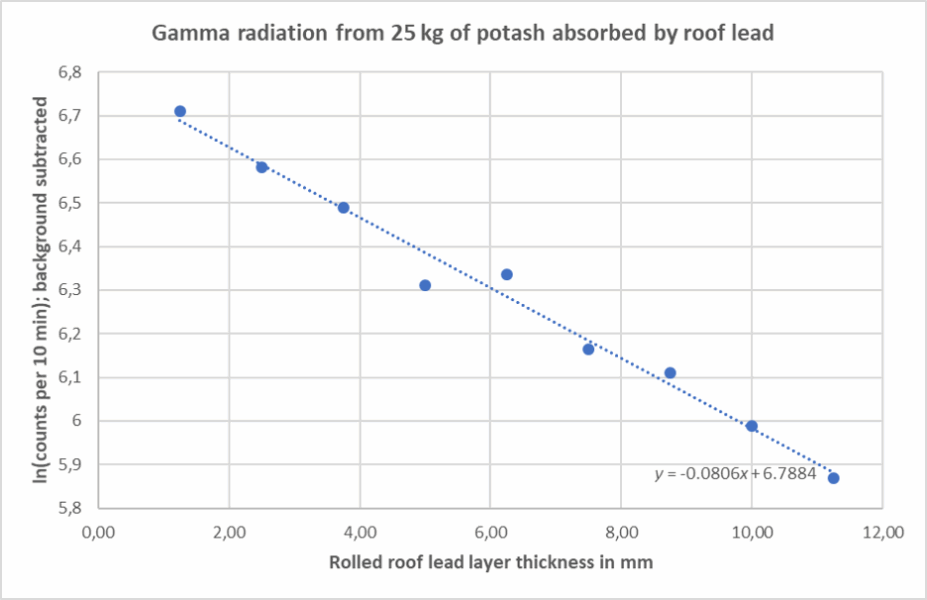
Exponential attenuation
Gamma radiation is attenuated as it passes through matter according to the Beer–Lambert law:
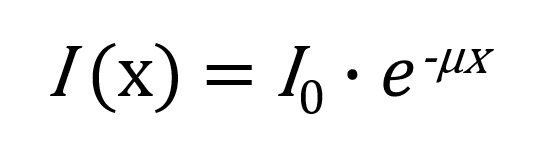
The value I (x ) gives the intensity after thickness x, and μ is the linear attenuation coefficient. The experimental data fits this law with μ ≈ 0.08 mm−¹ for lead. This allows us to calculate the half-thickness value:

Or the attenuation length:

These values agree with known data.[4]
Efficiency and limitations
Older students that already having some knowledge of radioactivity can estimate the half-life of potassium-40 based on measured count rates and known quantities of material. This activity introduces concepts such as specific activity, decay constants, and long-lived isotopes.
Full instructions for the determination of half-life activity can be found in the supporting material.
This series of experiments demonstrates that the core principles of radiation physics can be effectively taught using safe, accessible, and inexpensive materials. By using potassium carbonate – a simple food-grade compound rich in naturally occurring potassium-40 – students gain hands-on experience with beta and gamma radiation, shielding effects, the inverse square law, and the concept of half-life.
This model highlights that meaningful engagement with nuclear physics is not limited to specialized laboratories. With creativity and careful design, powerful learning experiences can be created using everyday materials – bringing radioactivity out of the shadows and into the light for student exploration.
[1] Strahlenschutzverordnung Deutschland 2017: https://www.gesetze-im-internet.de/strlschv_2018 (in German)
[2] Krieger H (2007) Grundlagen der Strahlungsphysik und des Strahlenschutzes. Teubner. ISBN: 9783835101999
[3] Wischnewski V, Schwanker RJ, Mueller HJ (1985). Zur Radiochemie von [40K]Kalium. PdN-Chemie 34: 32−38.
[4] Explanations of gamma attenuation: https://radioactivity.eu.com/articles/questions_of_doses/gamma_attenuation
Download this article as a PDF

You shall not pass: explore the function of deep geological repositories and the key role of bentonite in preventing the leakage of highly radioactive waste.

Helium: gas of awe, wonder, and worry. Is it time to give this noble gas the respect it…
Chasing rainbows: the interaction of an electric current and magnetic field in a solution with pH indicator gives amazing colour patterns as electrolysis occurs.