Supporting materials
Download
Download this article as a PDF

Stacking up: use common household items like coins and paper explore one of the most significant scientific discoveries of the 19th century.
The focus of this project is to learn about batteries by building small devices capable of generating electrical energy using common materials, such as coins, aluminium foil, and paper towels. This process will mirror the creation of Alessandro Volta’s famous voltaic pile from the early 1800s. A historical narrative on the debate between the Italian scientists Volta and Galvani sets the stage,[1–4] and the activities culminate with students building their own functional devices.

These activities are suitable for high school students aged 15–18.
This activity serves as an introduction to electrochemistry by exploring the historical debate between Alessandro Volta and Luigi Galvani on the nature of electricity. This not only helps students understand the scientific context of the time, but also highlights the evolving nature of scientific inquiry and the importance of evidence in shaping our understanding of the natural world.
The debate originated in the late 18th century when Luigi Galvani observed muscle contractions in frogs’ legs in response to electrical stimuli, leading him to propose the idea of ‘animal electricity’. Alessandro Volta, however, challenged this theory, arguing that the observed phenomenon was due to the contact between different metals, rather than an intrinsic property of the living tissue.
This activity takes approximately 90 minutes.
If time or suitable facilities are not available, the activity could be assigned as a home project, allowing students to work at their own pace while still following the suggested guidelines. Students are free to choose their own research materials and presentation formats.

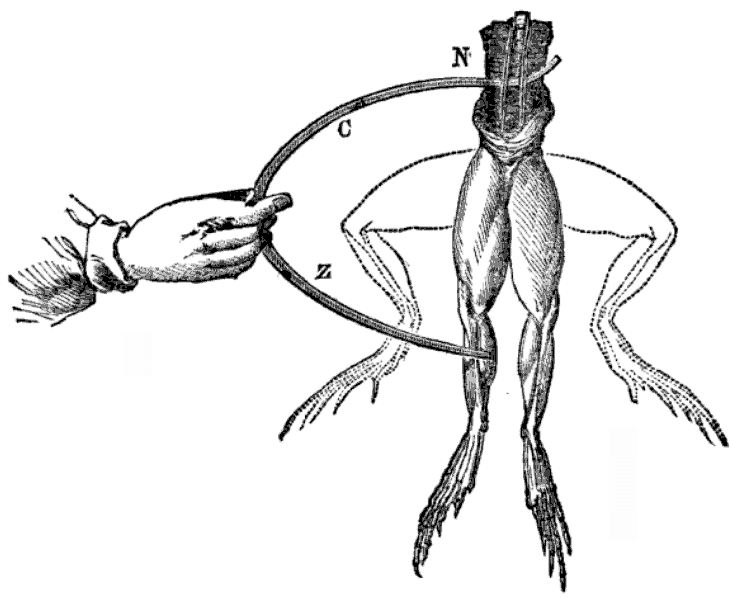
This approach offers a clear framework for students, while still allowing them the freedom to explore creatively.
In this activity, students are encouraged to argue the two sides of the historical debate in either a presentation or role-playing context. By engaging with the historical context and presenting arguments, students will develop critical thinking, public speaking, and collaboration skills, while gaining an appreciation for the foundations of electrochemistry.
The teacher can organize the discussion in one of two formats, depending on class size and objectives: a scientific talk or a structured debate. Both methods emphasize active learning, debate, and inquiry-based exploration.
This activity takes approximately 90 minutes, including research, debate preparation, and discussion
After the debate, the teacher can clarify how, in light of modern scientific understanding, both Volta and Galvani had valid perspectives, illustrating how different viewpoints can reveal multiple aspects of the same phenomenon. The Galvani and Volta infosheet can be used as a basis for this.
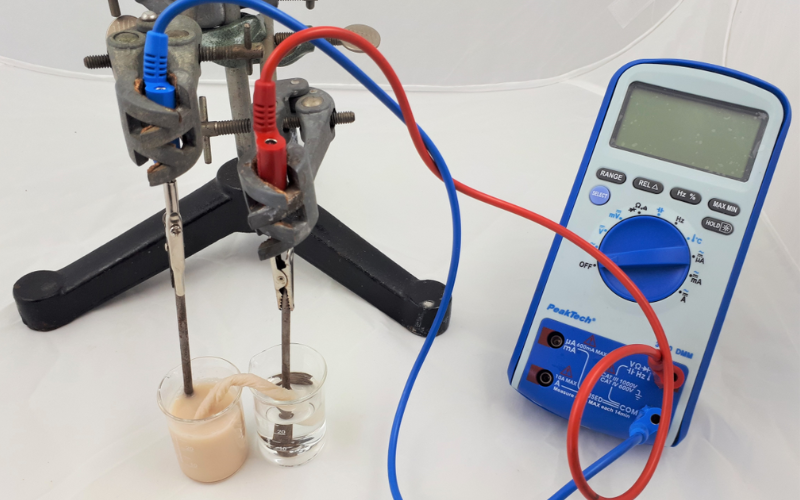
In this activity, students explore the basics of electrochemistry by constructing a simple voltaic pile using coins, aluminium foil, and paper towels. This experiment allows students to understand how chemical energy is converted into electrical energy, laying the foundation for more advanced discussions about batteries and energy storage.
Learning objectives
– Understand the principles behind the voltaic pile and redox reactions.
– Gain practical skills in assembling and testing an electrochemical cell.
– Recognize the connections between historical scientific discoveries and modern technologies.
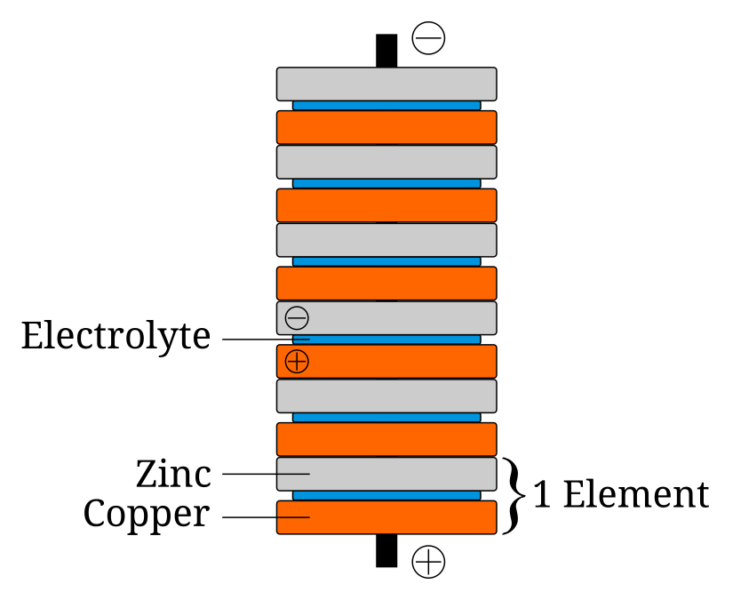
The activity takes approximately 60 minutes: 20 minutes for preparation and setup, 20 minutes for the construction and testing of the pile, and 20 minutes for discussion and measurements.
While this activity uses euro 5 cent coins for their copper content, any small copper coins can be used as a substitute. For example, pennies from the USA or UK; preferably those minted before 1992 in the UK or before 1982 in the USA, as they contain a higher percentage of copper. The key is to ensure that the coins have a sufficient copper surface to act as the cathode in the voltaic pile.


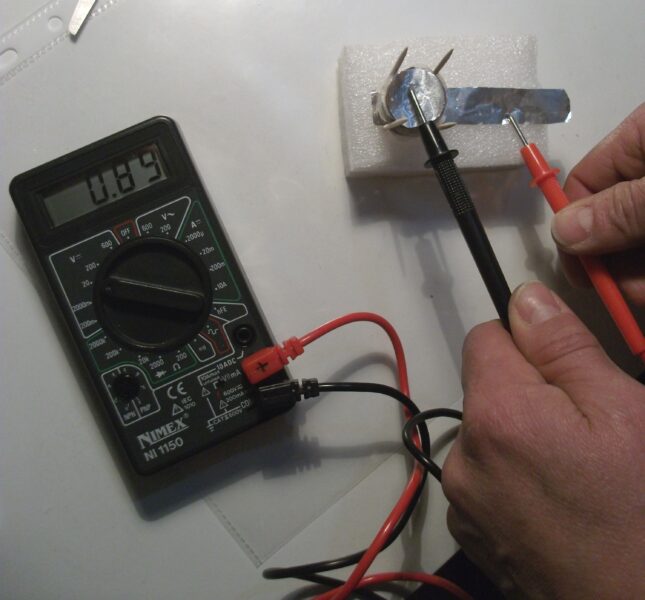

To help students start thinking about the fundamental principles shared by all batteries, the teacher can introduce some leading questions:
Through discussion and guided exploration, students will uncover that the darkening of the coins is due to chemical reactions at the electrode surfaces, specifically oxidation. This observation reinforces the idea that redox reactions are at the core of how batteries generate electricity.
A battery converts chemical energy into electrical energy through spontaneous redox reactions.
The following are essential elements for electricity production:
Anode (−): site of oxidation (loss of electrons)
Cathode (+): site of reduction (gain of electrons)
Salt bridge: contains an electrolyte that facilitates the free movement of positive and negative ions
Electrons flow from the anode to the cathode via a conductive wire.
The composition of the battery constructed is as follows: anode, aluminium; cathode, oxygen; electrolyte, water and NaCl solution; this is the representation of a prototype of an air/aluminium battery.[6]
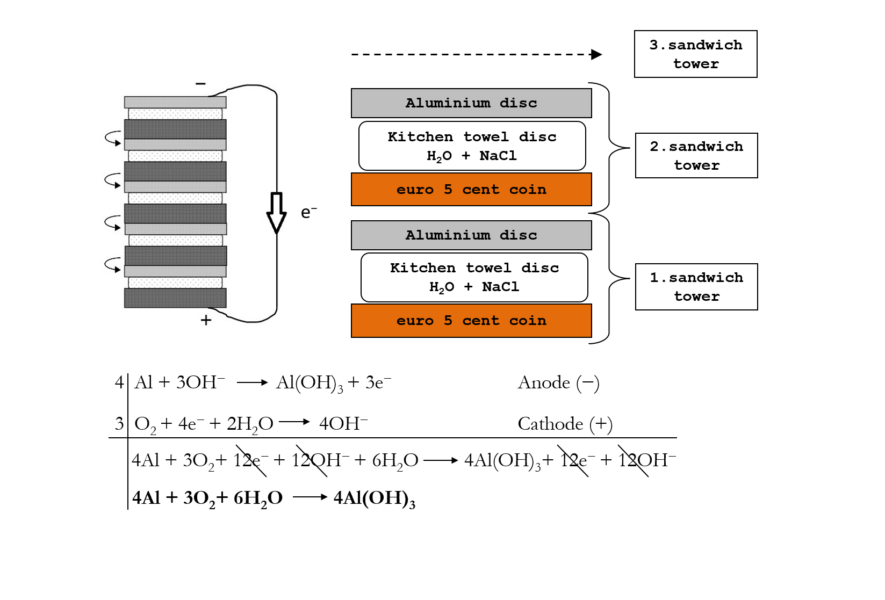
It is essential to highlight that, although Alessandro Volta invented a device that revolutionized science and technology, he was never able to provide an effective explanation of how it worked. This is understandable, given that the discovery of the electron was still 100 years away. Despite this, modern batteries, even the most complex, operate on the same basic mechanism, involving an anode, a cathode, and a salt bridge.
Constructing a voltaic pile provides a wealth of opportunities for reflection and to link to curriculum topics.
The primary discipline involved is electrochemistry, focusing on core concepts such as energy transformation, oxidation–reduction reactions, and electrical circuits. Additionally, the invention of the voltaic pile followed shortly after Galvani’s death and was a significant development in the broader scientific debate about the nature of animal electricity. The research conducted by Volta and Galvani laid the foundation for modern electrophysiology.
The voltaic pile was crucial for chemistry, enabling the decomposition of numerous substances (electrolysis) and leading to the discovery of new chemical elements, such as the separation of water into hydrogen and oxygen. In physics, the discovery of the magnetic effects of an electric current paved the way for the invention of electric motors and dynamos (Faraday) and the formulation of electromagnetic field theory (Maxwell). The voltaic pile enabled countless scientific explorations, leading to devices such as light bulbs, dynamos, telegraphs, radios, and radars. Furthermore, in 1818, Mary Shelley published Frankenstein, which tells the story of an inanimate creature brought to life by electrical discharges, likely inspired by the Volta–Galvani debate. The themes of this work are still relevant. Should science have limits? What is life? Can death be conquered? Literature, therefore, serves as a means to decode reality, including scientific realities.
[1] Piccolino M, Bresadola M (2003) Rane, Torpedini e scintille. BollatiBoringhieri. ISBN: 9788833914701 (Italian language)
[2] Piccolino M (2000) ‘Anno 2000: Volta elettrofisiologo 200 annidopol’invenzionedella pila voltaica. Naturalmente 13: 6–15. (Italian language)
[3] An article on the discovery of bioelectricity: https://cerebromente.org.br/n06/historia/bioelectr2_i.htm
[4] A summary of the debate between the Italian scientists Volta and Galvani: https://www.whipplemuseum.cam.ac.uk/explore-whipple-collections/frogs/frogs-and-animal-electricity
[5] Simple experiments with a Volta battery (Italian language): https://www.reinventore.it/approfondimenti/scatolab-10-la-pila-di-volta
[6] Bharti R et al (2024) Aluminum–air batteries: current advances and promises with future directions. RSC Advances 14: 17628–17663. doi: 10.1039/d4ra02219j
Download this article as a PDF

Pocketful of sunshine: build a solar cooker and learn about the thermoelectric effect with Peltier…
Explore the everyday science behind the quest to harness fusion energy – the energy that powers the stars – in a safe way here on Earth.
Redox reactions carried out by inexpensive baker’s yeast during breadmaking can also be used to demonstrate biofuel cells in the classroom.