Supporting materials
Download
Download this article as a PDF

Redox reactions carried out by inexpensive baker’s yeast during breadmaking can also be used to demonstrate biofuel cells in the classroom.
The energy transition – from producing energy through burning fossil fuels to using renewable sources – is one of the main global challenges of this century. As fuel cells can play a key role in the energy transition, they should be an integral part of chemistry education. In addition to hydrogen cells, other fuel and catalyst systems can be used, including biofuel cells, thus opening up a wide range of applications. Biofuel cells also intersect with many curriculum science topics spanning different science subjects, including enzymes, metabolism, microbiology, redox reactions and electrochemistry, and energy production. Thus, they provide an excellent opportunity to deepen an understanding of these topics in a real-world context and to demonstrate the importance of multidisciplinary approaches in science.
If microorganisms or enzymes are used as catalysts in biofuel cells, the systems are referred to as biological fuel cells. They are operated under mild conditions, such as normal pressure and temperatures between 4 and 37°C. Their setup is often realized as a two-chamber system.
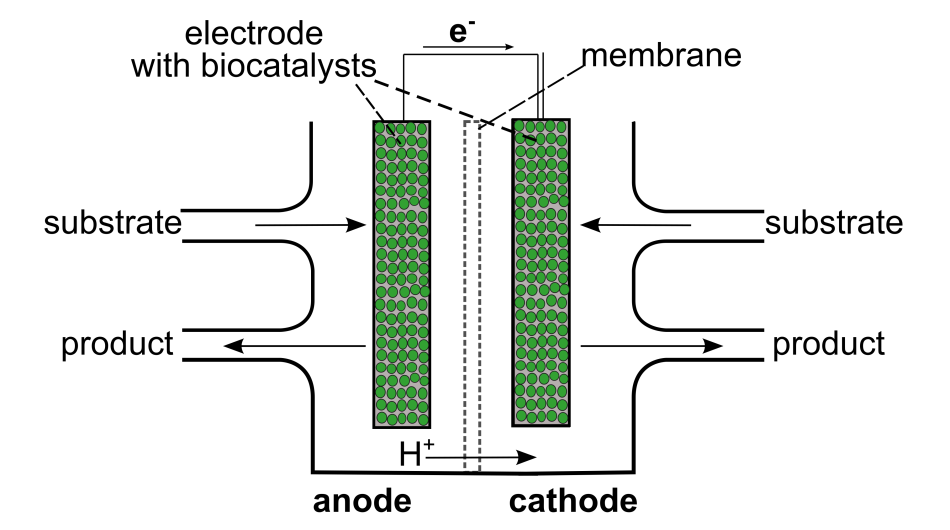
The fuel, referred to here as the substrate, is fed to the anode side and oxidized. If an inorganic oxidant is used at the cathode side, it is also referred to as a substrate. For continuous operation of the cell, the respective reactants have to be fed into the cell and the products removed continuously. The catalysts can either be immobilized on an electrode or distributed in the surrounding solution. In microbial fuel cells (MFCs), a great range of substrates can be used, even waste materials. The main area of application is thus wastewater treatment with simultaneous generation of electricity.[1,2]
Biological fuel cells, and therefore, MFCs, are currently underrepresented in schools. In the (German) curricula, only hydrogen–oxygen fuel cells, which are currently the most widespread kind of fuel cells, are addressed. In this article, we focus on baker’s yeast and its application as a biocatalyst suitable for school. Since this biocatalyst can be cheaply purchased in the supermarket, the experimental setups are low cost and nontoxic. Furthermore, students may already be familiar with the metabolism of yeast from their everyday lives, so the working principle of these fuel cells can be easily understood during experiments. The experiments are aimed at students aged 11 and older. They can explore how baker’s yeast can be used as a biocatalyst (Activity 1) and how a fuel cell works in general (Activity 2). Activity 2 is to be understood as a model experiment for a biological fuel cell, with the limitation that there is no continuous supply of substrate and withdrawal of product.
This simple experiment shows that electrons are released during the metabolism of baker’s yeast. For this purpose, the redox indicator methylene blue is used. By accepting electrons, methylene blue turns from blue into its colourless leuco form.
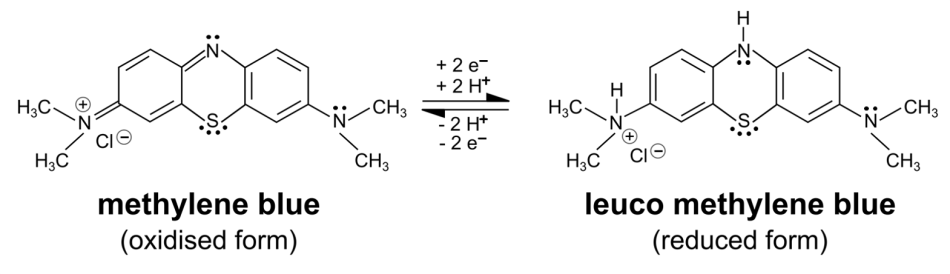
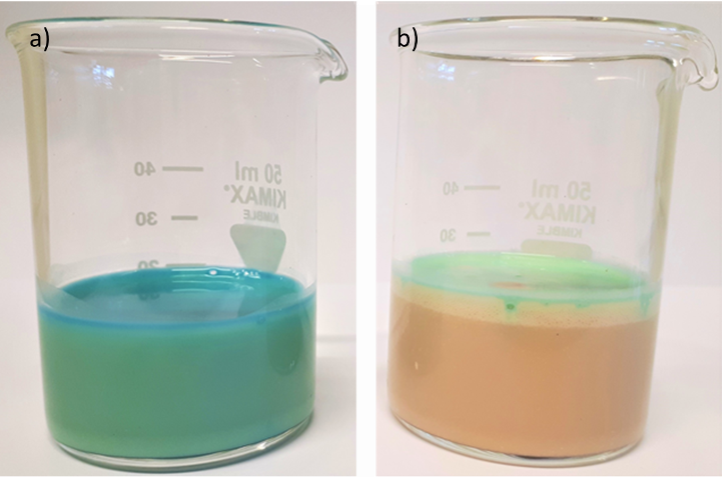
This activity can be seen as an introduction to the operating principle of a microbial fuel cell. Illustrated instructions for Activity 1 are available in the supporting material. The basic version of this activity takes about 15 minutes. The experiment can be repeated at different temperatures to illustrate the temperature dependence of metabolic processes. For example, at 30°C, decolourization takes less time because it is closer to the optimum temperature of metabolism than room temperature.
After a few minutes, in the initially blue suspension, the formation of colourless gas and foam can be observed along with decolourization.
The following questions can be used to lead a discussion of the experiment:
A model answer sheet is provided in the supporting material.
In the system described below, baker’s yeast works as a biocatalyst. The microbial fuel cell´s setup is kept simple and reduced to only necessary components: The two-pot structure is very easy to approach, as it is similar to well-known galvanic cells. Illustrated instructions for Activity 2 are provided in the supporting material.
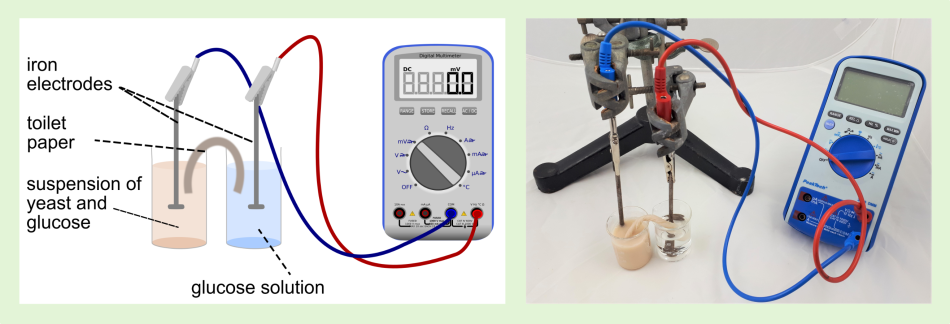
It takes about 20 minutes to build and operate the cell.
The potential difference, and hence, the measurable voltage are caused by the yeast’s metabolic activity in the anode compartment. In this setup, at a temperature of 20°C, a voltage of up to 400 mV is measured. With decreasing concentration of substrate in the anode compartment and a decreasing pH, the yeast’s metabolic activity decreases, so that the voltage declines and finally drops off. The current measured after 10 minutes is about 30 µA. It is not sufficient to operate a load.
Simplified reactions for the metabolism with glucose as a substrate can be formulated for the school context, as shown below:
If sucrose is used, it is split into glucose and fructose. Both are oxidized, finally yielding carbon dioxide, which can be observed as gas bubbles. Glucose is the yeast’s preferred substrate because no hydrolysis is needed, and it can be directly converted in the glycolysis metabolic pathway. Therefore, when using glucose, the voltage can be measured immediately. If sucrose is used, it takes a moment for the voltage to increase due to the necessary formation of the monosaccharides. Oxygen works as the final electron acceptor within the respiration chain. This is shown by the cathode reaction.
The following questions can help to discuss the observations and to understand the underlying processes:
A model answer sheet is provided in the supporting material.
By carrying out both activities, students learn about the basic working principle of microbial fuel cells. An even more compact one-pot setup with baker’s yeast as a biocatalyst has been developed for the school context.[3] The performance is higher than in the two-pot setup, but still too low to operate a small motor. It would be particularly valuable to set these experiments in the context of the energy transition, maybe in collaboration with the physics department. Students could research different types of fuel cells and possible applications, or make a comparison of different energy-production technologies.
Further materials on different kinds of microbial fuel cells are available (in German) from the chemistry education department of the University of Wuppertal,[4] or can be requested from the author. Materials are continuously being translated into English.
[1] Santoro C et al. (2017) Microbial fuel cells: from fundamentals to applications. A review. Journal of Power Sources 356: 225–244. doi: 10.1016/j.jpowsour.2017.03.109
[2] Kumar R et al. (2018) Microbial fuel cell is emerging as a versatile technology: a review on its possible applications, challenges and strategies to improve the performances. International Journal of Energy Research 42: 369–394. doi: 10.1002/er.3780
[3] Grandrath R, Bohrmann-Linde C (2019) Teaching sustainability in the chemistry classroom: exploring fuel cells in simple hands-on experiments with hydrogen, sugar and alcohol. World Journal of Chemical Education 7: 172–178. doi: 10.12691/wjce-7-2-17
[4] Website of the working group on chemistry education in Wuppertal: https://chemiedidaktik.uni-wuppertal.de/index.php?id=4859&L=0
Download this article as a PDF
How do social drugs affect metabolism? How is toxicity measured? How does climate change affect water ecosystems? Promote active learning by…

Can we meet all our energy needs with renewables? How can energy models help us to explore the future of energy? And how can we all become part of…

Pocketful of sunshine: build a solar cooker and learn about the thermoelectric effect with Peltier…