Food that shapes you: how diet can change your epigenome Understand article
You are what you eat – quite literally. Our diet can influence the tiny changes in our genome that underlie several diseases, including cancer and obesity.
Image courtesy of mstroeck /
Wikimedia Commons
When you look at yourself in the mirror you may ask, ‘How, given that all the cells in my body carry the same DNA, can my organs look so unlike and function so differently?’ With the recent progress in epigenetics, we are beginning to understand. We now know that cells use their genetic material in different ways: genes are switched on and off, resulting in the astonishing level of differentiation within our bodies.
Epigenetics describes the cellular processes that determine whether a certain gene will be transcribed and translated into its corresponding protein. The message can be conveyed through small and reversible chemical modifications to chromatin (figure 1). For example, the addition of acetyl groups (acetylation) to DNA scaffold proteins (histones) enhances transcription. In contrast, the addition of methyl groups (methylation) to some regulatory regions of the DNA itself reduces gene transcription. These modifications, together with other regulatory mechanisms, are particularly important during development – when the exact timing of gene activation is crucial to ensure accurate cellular differentiation – but continue to have an effect into adulthood.
Epigenetic modifications can occur in response to environmental stimuli, one of the most important of which is diet. The mechanisms by which diet affects epigenetics are not fully understood, but some clear examples are well known.
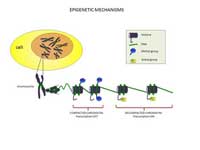
to the chromatin structure
involve mainly histone
acetylation – which enhances
transcription – and DNA
methylation, whereby methyl
groups are covalently bound
to cytosine, making the
chromatin structure less
accessible. These changes are
reversible, allowing gene
activity to be adapted to
changing environmental
conditions or signals.
This image was updated on the
13 May 2014.
Image courtesy of Cristina Florean
During the winter of 1944–1945, the Netherlands suffered a terrible famine as a result of the German occupation, and the population’s nutritional intake dropped to fewer than 1000 calories per day. Women continued to conceive and give birth during these hard times, and these children are now adults in their sixties. Recent studies have revealed that these individuals – exposed to calorie restrictions while in their mother’s uterus – have a higher rate of chronic conditions such as diabetes, cardiovascular disease and obesity than their siblings. The first months of pregnancy seem to have had the greatest effect on disease risk.
How can something that happened before you were even born influence your life as much as 60 years later? The answer appears to lie in the epigenetic adaptations made by the foetus in response to the limited supply of nutrients. The exact epigenetic alterations are still not clear, but it was discovered that people who were exposed to famine in utero have a lower degree of methylation of a gene implicated in insulin metabolism (the insulin-like growth factor II gene) than their unexposed siblings (Heijmans et al., 2008). This has some startling implications: although epigenetic changes are in theory reversible, useful changes that take place during embryonic development can nonetheless persist in adult life, even when they are no longer useful and could even be detrimental. Some of these changes may even persist through generations, affecting the grandchildren of the exposed women (Painter et al., 2008).

honeybee larvae floating in
royal jelly in their queen cell.
Queen larvae are fed
exclusively with royal jelly,
which triggers the
development of the queen
phenotype, allowing
reproduction
Image courtesy of Waugsberg /
Wikimedia Commons
The effects of early diet on epigenetics are also clearly visible among honeybees. What differentiates the sterile worker bees from the fertile queen is not genetics, but the diet that they follow as larvae (figure 2). Larvae designated to become queens are fed exclusively with royal jelly, a substance secreted by worker bees, which switches on the gene programme that results in the bee becoming fertile.
Another striking example of how nutrition influences epigenetics during development is found in mice. Individuals with an active agouti gene have a yellow coat and a propensity to become obese. This gene, however, can be switched off by DNA methylation. If a pregnant agouti mouse receives dietary supplements that can release methyl groups – such as folic acid or choline – the pups’ agouti genes become methylated and thus inactive. These pups still carry the agouti gene but they lose the agouti phenotype: they have brown fur and no increased tendency towards obesity (figure 3).
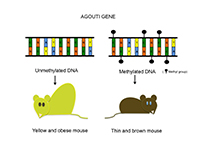
model. The phenotype
depends on the mother’s diet
during pregnancy. A:
Normally, the agouti gene is
associated with yellow fur
and a tendency towards
obesity. B: Mice born to a
mother receiving dietary
supplements of methyl
donors, however, have a
methylated and thus
inactivated agouti gene,
resulting in a thin, brown-
fur phenotype.
Image courtesy of Cristina
Florean
An insufficient uptake of folic acid is also implicated in developmental conditions in humans, such as spina bifida and other neural tube defects. To prevent such problems, folic acid supplements are widely recommended for pregnant women and for those hoping to conceive (see Hayes et al., 2009).
What about the dietary effect on epigenetics in adult life? Many components of food have the potential to cause epigenetic changes in humans. For example, broccoli and other cruciferous vegetables contain isothiocyanates, which are able to increase histone acetylation. Soya, on the other hand, is a source of the isoflavone genistein, which is thought to decrease DNA methylation in certain genes. Found in green tea, the polyphenol compound epigallocatechin-3-gallate has many biological activities, including the inhibition of DNA methylation. Curcumin, a compound found in turmeric (Curcuma longa), can have multiple effects on gene activation, because it inhibits DNA methylation but also modulates histone acetylation. Figure 4 shows further examples of epigenetically active molecules.

Image courtesy of Marcel
Theisen / Wikimedia Commons
Most of the data collected so far about these compounds come from in vitro experiments. The purified molecules were tested on cellular lines, and their effects on epigenetic targets were measured. It remains to be proved if eating the corresponding foods has the same detectable effect as has been seen in cellular models (Gerhauser, 2013).
Epidemiological studies, however, suggest that populations that consume large amounts of some of these foods appear to be less prone to certain diseases (Siddiqui et al., 2007). However, most of these compounds not only have epigenetic effects but also affect other biological functions. A food may contain many different biologically active molecules, making it difficult to draw a direct correlation between epigenetic activity and the overall effect on the body. Finally, all foods undergo many transformations in our digestive system, so it is not clear how much of the active compounds actually reach their molecular targets.
As a result of their far-reaching effects, epigenetic changes are involved in the development of many illnesses, including some cancers and neurological diseases. As cells become malignant, or cancerous, epigenetic modifications can deactivate tumour suppressor genes, which prevent excessive cell proliferation (Esteller, 2007). Because these epigenetic modifications are reversible, there is great interest in finding molecules – especially dietary sources – that might undo these damaging changes and prevent the development of the tumour.
We all know that a diet rich in fruit and vegetables is healthy for our everyday life, but it is becoming increasingly clear that it might be much more important than that, having significant implications for our long-term health and life expectancy.
References
- Esteller M (2007) Epigenetic gene silencing in cancer: the DNA hypermethylome. Human Molecular Genetics 16(1): R50-R59. doi:10.1093/hmg/ddm018
- Gerhauser C (2013) Cancer chemoprevention and nutri-epigenetics: state of the art and future challenges. Topics in Current Chemistry 329: 73-132. doi:10.1007/128_2012_360
- Hayes E, Maul H, Freerksen N (2009) Folic acid: why school students need to know about it. Science in School 13: 59-64.
- Heijmans BT et al. (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the USA 105: 17046-17049. doi:10.1073/pnas.0806560105
- Painter R et al. (2008) Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG: An International Journal of Obstetrics & Gynaecology 115: 1243-1249. doi:10.1111/j.1471-0528.2008.01822.x
- Siddiqui IA et al. (2007) Tea beverage in chemoprevention and chemotherapy of prostate cancer. Acta Pharmacol Sinica 28(9): 1392-1408. doi:10.1111/j.1745-7254.2007.00693.x
Resources
- For a simple introduction to epigenetics, see:
- McVittie B (2006) Epigenetics. Science in School 2: 62-64.
- To learn more about nutrition and epigenetics, see:
- Link A et al. (2010) Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochemical Pharmacology 80(12): 1771- 1792. doi:10.1016/j.bcp.2010.06.036
- The Learn Genetics website.
- For more information about the effect of the Dutch famine on adult life and gene methylation, see:
- Roseboom TJ et al. (2001) Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Molecular and Cellular Endocrinology 185: 93-8. doi:10.1016/S0303-7207(01)00721-3
- The website of the University of Leiden
- For a fascinating and very readable explanation of some recent research into honeybee epigenetics, see:
- Chittka A, Chittka L (2010) Epigenetics of royalty. PLOS Biology 8(11): e1000532. doi:10.1371/journal.pbio.1000532.
- PLOS Biology is an open-access journal, so this article is freely available online.
- For more information about honeybee epigenetics
- For a simple overview of epigenetics and the agouti gene in mice, see:
- Adams J (2008) Obesity, epigenetics, and gene regulation. Nature Education 1(1).
- To learn how hormone levels during pregnancy can affect the sex of the child, see:
- Notman (2012) Intersex: falling outside the norm. Science in School 23: 48-52.
Review
The article establishes a link between diet during pregnancy and changes in the expression of genes due to the mechanisms of histone acetylation (enhancing transcription) and methylation (reducing transcription). Using examples in humans, mice and honeybees, the article shows that the lack of certain nutrients can affect the development of traits in children. It also deals with dietary effects on epigenetics in adult life, listing a number of foods that are known to have a positive influence on health.
This article could be used as the basis for a discussion about healthy dietary choices compared with junk food, in order to increase students’ awareness of the possible consequences of their eating behaviour.
The article could be used in a lesson reviewing some basic topics about gene expression.
Potential questions could include:
- What is the structure and function of histones?
- What are the main mechanisms of regulation of gene expression?
- How does genotype affect phenotype expression?
- How do environmental conditions (internal or external) influence gene expression?
- Diabetes is a good example of a disease that is linked to diet. Can you describe the causes of diabetes?
Monica Menesini, Liceo Scientifico Vallisneri Lucca, Italy





