Indigo: recreating Pharaoh’s dye Teach article
What links your jeans, sea snails, woad plants and the Egyptian royal family? It’s the dye, indigo. Learn about its fascinating history and how you can extract it at school.

plant leaves
Image courtesy of gitane;
image source: Wikimedia
Commons
In ancient Egypt, only one boat had a purple-dyed sail. It belonged to the Pharaoh and was a vibrant and powerful sign to other Nile users that they should move aside to let the royal boat pass.
Even today, deep blue, purple and crimson are traditionally associated with royalty, luxury and wealth. This is because they are difficult and expensive colours to achieve using natural dyes. Until the advent of synthetic dyes 100 years ago, natural dyes (from plant, animal or mineral sources) were the only way to colour fabrics.

Egyptian royal boat, with a
furled imperial purple sail.
Note that ‘purple’ is not
always what we would
expect today. Click on image
to enlarge
Image courtesy of dudchik /
iStockphoto
The vivid purple of the Pharaoh’s sail was achieved using indigo – a natural dye that can be extracted from certain plants and animals. Thanks to Roman naturalist Pliny the Elder (see box on Pliny the Elder), we even know the dyeing methods used by ancient civilisations. This activity allows your students to follow in the footsteps of these early chemists, extracting indigo from the leaves of the woad plant.
Using basic lab equipment, secondary students of all ages could carry out this extraction in one or two practical sessions.
Younger students (ages 11-15) could simply do the extraction without going into much detail about the chemical reaction [although note that the reviewer suggested the activity was suitable for students aged 14+]. More advanced organic chemistry students (ages 16+) could investigate the compounds and reactions more thoroughly. The experiment is also relevant to the use of science in industry and could be used as part of a project on this topic.
All about indigo
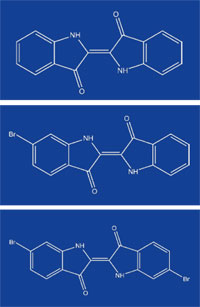
(top), mono-bromoindigo
(middle) and di-bromoindigo
(bottom). Click on image to
enlarge
Images courtesy of Gianluca
Farusi
Indigo is an organic compound that is found in three differently coloured forms – indigo itself, which is blue; purple mono-bromoindigo; and red-purple di-bromoindigo (figure 1). Naturally derived dyes can contain just one of these pigments or a mixture of two or three in variable proportions, leading to a range of colours from red to blue (Cooksey, 2001). The intensity of the colour is also influenced by the way it is processed, such as whether the dyed cloth is dried in the light or shade.
In ancient Egyptian times, the best quality indigo was extracted from Murex sea snails, which were once common in the coastal waters of the eastern Mediterranean. Archaeological evidence from Crete suggests that indigo extraction from Murex snails had begun by 2000 BC. By 1000 BC the Phoenicians, a civilisation centred in what is now Lebanon, were exploiting this valuable dye.

brandaris the secretions
of which were first used
to make indigo in 2000 BC
Image courtesy of H Zell;
image source: Wikimedia
Commons
Their entire economy was based on trading Tyrian purple, a violet-purple indigo dye derived from the snail Murex brandaris (now known as Bolinus brandaris). They were so famed for it that the name Phoenician is derived from the Greek word ‘phoin?sso’, meaning ‘to make red’.
Murex-derived indigo was so expensive because 12 000 snails yield just 1.4 g Tyrian purple – enough to dye a handkerchief! The compounds used to make the dyestuff are secreted by the snail’s hypobranchial gland, found between the bowels and the branchial organ. The snails appear to produce these indigo precursors as a defence response and for their antimicrobial properties. The fresh secretions are colourless, but darken when exposed to air. According to Pliny the Elder, the dye was extracted by crushing the snails, leaving them to putrefy for three days in alkaline salt water, and then boiling them for up to ten days – imagine the smell!
The Phoenicians perfected this process over hundreds of years and discovered that by varying the species of snail, extraction methods and processing, they could produce a range of colours from red to purple to blue (table 1).
| Species | Appearance | Dye precursors found in the organism | Dye name | Colour | Chemical composition of dye |
|---|---|---|---|---|---|
| Bolinus brandaris (formerly Murex brandaris) |
|
6-bromoindoxyl | Tyrian purple / imperial purple / argaman | Red-purple | Mainly di-bromoindigo |
| Hexaplex trunculus (formerly Murex trunculus) |
|
Indoxyl and 6-bromoindoxyl | Royal blue / tekhelet | Blue-purple | Mixture of indigo, mono- and di-bromoindigo |
| Indigofera tinctoria (true indigo) |
|
Indican | Indigo | Blue | Indigo |
| Isatis tinctoria (woad) |
|
Isatan B | Indigo | Blue | Indigo |
Lower-quality indigo can also be extracted from certain plants, and this technique actually pre-dates the Phoenicians’ snail-derived dye. In India, methods of extracting indigo from the ‘true indigo’ shrub Indigofera tinctoria were known before 2000 BC. In Europe, the dye was extracted from woad (Isatis tinctoria). Although these two plants contain different precursors, indican in true indigo and isatan B in woad (table 1 and figure 2), they both ultimately yield the same dye – indigo.
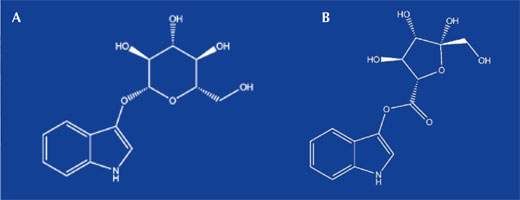
Images courtesy of Gianluca Farusi
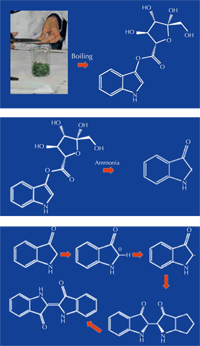
conversion of isatan B to
indigo. Click on images to
enlarge
Images courtesy of Gianluca
Farusi
The production of indigo from woad takes place in three main stages:
- Boiling the leaves to extract the water-soluble isatan B (figure 3A; steps 1-6 in the activity below).
- Adding alkali (ammonia) to hydrolyse the molecule, removing the carbohydrate group to leave indolin-3-one or its tautomer, indoxyl (figure 3B; step 7 in the activity below).
- Exposing the extract to the air, leading to the oxidation and coupling of two indolin-3-one molecules to form the blue-coloured indigo (figure 3C; dyeing the wool and obtaining indigo powder in the activity below).
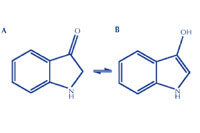
Click on image to enlarge
Image courtesy of Gianluca Farusi
Student activity: extracting indigo from woad leaves
Materials
image source: Wikimedia
Commons
For each group of students:
- Fresh woad (Isatis tinctoria) leaves (enough to fill a 1 l beaker)
- Woad seeds are easily obtainable from garden centres, and in some countries woad is a very common plant.
- 20 wt% ammonia solution
- Three 1 l beakers
- Distilled water
- Octanoic acid (optional)
- Bunsen burner and tripod
- Stirring rod
- Sieve or strainer
- Measuring cylinders and/or 10 ml pipettes
- Heatproof gloves
- Funnel and filter paper
- Knife for cutting the leaves (optional)
- Wool for dying (optional)
I used a ball of white knitting wool, and some rough grey-white sheep’s fleece.
Procedure
- Fill a beaker with woad leaves. Remove the leaves from the beaker and cut or tear into small pieces.
- Fill the beaker two-thirds full with distilled water and bring to the boil over a Bunsen burner.
-

Indigo-dyed linen from 1873
Image courtesy of PKM; image
source: Wikimedia CommonsAdd a few torn leaves to the boiling water, stir and continue heating. When the water has returned to the boil, add a few more leaves. Continue adding leaves and heating until all the woad has been added.
- When all the leaves have been added, continue boiling for 1 minute.
- Strain all the liquid from the boiled leaves into a clean beaker.
- Cool the extract to room temperature by floating the beaker in a shallow tray full of cold water, stirring both the extract in the beaker and the water in the tray. You now have a solution containing, among lots of other things, isatan B, which is an unstable glycoside of indoxyl.
- When cool, measure the volume of your extract and add ammonia equivalent to 1 % of that volume, e.g. for 1 l extract, add 10 ml ammonia; for 100 ml extract, add 1 ml ammonia. The solution should immediately change from brown to brown-yellow then green. You now have a solution containing, among lots of other things, indoxyl.
To dye wool
Wet the wool and place in the extract for 10 minutes. Remove and leave to air dry.

background of woad from
which it was extracted
Image courtesy of naturaldyer;
image source: Flickr
To obtain indigo powder
- Aerate the extract by pouring the solution from one beaker to another for 10 minutes.
- Optional: if too much foam develops during aeration, add some octanoic acid, drop by drop. This acts as a surfactant.
- Filter the extract through ordinary filter paper to obtain the indigo powder.
To use the indigo powder as a dye, it needs to be dissolved in water and mixed with a reducing agent, such as sodium hydrosulphite (Na2S2O4). When the dyed material (e.g. wool) is exposed to air, it will turn blue.
Safety note
Remember that indigo and many of its precursors are dyes, so take care not to spill them on clothes or skin. When handling concentrated ammonia solution, as well as indigo and its precursors, wear gloves and chemical splash glasses, and use a fume hood. See also the general Science in School safety note.
Questions for discussion
- Why do we boil the leaves? What is happening?
- What happens when the ammonia is added?
- When you dye the wool, why does the blue colour appear as the wool dries?
- When you produce the powder, what is happening when the solution is poured from beaker to beaker? Why does the indigo precipitate?
- Can you find out how indigo is produced industrially today? What are its major uses?
Extension activities
You could also ask your students to further investigate the chemistry of indigo and other dyes with some of the following activities.
Calculating the yield of indigo
Weigh the leaves before the extraction and the dried indigo powder. Calculate the percentage yield of indigo as follows: (mass of indigo / mass of leaves) x 100. You could compare the yield from fresh and dried leaves, or compare it with that of other indigo sources such as Indigofera tinctoria.

image source: Flickr
Using indigo as a dye
Our experiment involves using indigo to dye wool. Can indigo be used to dye other materials? You could compare natural materials such as cotton, linen and silk with synthetics or synthetic mixes (e.g. polyester cotton, cotton lycra).
The effectiveness of indigo production
- Which produces more indigo, larger, older leaves or younger, smaller leaves? Express the amount of indigo obtained by weight and per leaf (count leaves before extracting them). Which produces the most indigo?
- Grow plants with and without added nitrogen fertiliser. Aim for 2-4 g nitrogen per m2 of soil (you will need to calculate the amount of fertiliser that gives this amount of nitrogen). Does adding nitrogen fertiliser increase the amount of indigo produced?
- Try putting the plants in the dark for a few days (cover them with black plastic). Does this have an effect on the amount of indigo produced? What can you conclude about the metabolism of isatan B in the plant?
- Investigate the effect of other treatments to the plants or the cut leaves.
- Test the effect of varying the method of the extraction, for example using different kinds of alkali.

Click on image to enlarge
Image courtesy of Marcel Douwe Dekker; image source: Flickr
Investigating other dyes
My students produced dyes from onion skins, from madder (Farusi, 2006), and from myrtle berries. In addition, they tested how colour-fast their dyes were by washing the dyed materials using different methods.
Studying chemistry with Pliny the Elder

Naturalis Historia. Click on
image to enlarge
Image courtesy of PHGCOM;
image source: Wikimedia
Commons
This activity is part of a larger interdisciplinary project, developed together with 14- to 15-year-old students, to explore ancient scientific techniques. Pliny the Elder (23-79 AD) was a Roman author and naturalist. His encyclopaedia, Naturalis Historia, brings together much of the scientific knowledge of the time. We began each topic by discussing a passage from Naturalis Historia and then worked out how to recreate either the experiment described in the text or something similar.
In this way, the students began in the same pre-scientific state as Pliny and, through laboratory work and discussion, gained modern scientific knowledge on each of the topics. The process motivated even the most unenthusiastic students.
Other activities in the project include recreating ancient perfumes (Farusi, 2011), preparing glass tesserae with boric acid, simulating the luminescence of the shellfish Pholas dactylus, and preparing iron-gall ink (Farusi, 2007).
Acknowledgement
The author would like to thank Dr David Hill from the University of Bristol, UK, for his tips on extraction of indigo from woad.
References
- Cooksey CJ (2001) Tyrian purple: 6,6’-dibromoindigo and related compounds. Molecules 6: 736-769.
- Farusi G (2006) Teaching science and humanities: an interdisciplinary approach. Science in School 1: 30-33.
- Farusi G (2007) Monastic ink: linking chemistry and history. Science in School 6: 36-40.
- Farusi G (2011) Smell like Julius Caesar: recreating ancient perfumes in the laboratory. Science in School 21: 40-46.
Resources
- Seefelder M (1994) Indigo in culture, science and technology. Landsberg, Germany: Ecomed Verlagsgesellschaft. ISBN 978-3609651606
Review
In this and several other Science in School articles, Gianluca Farusi successfully blends ancient history and chemistry in an unusual mixture – in this case, an innovative and simple practical activity to isolate the dye indigo from the leaves of the woad plant (Isatis tinctoria). In my experience, practical activities involving intense colours are an effective way to attract the students’ attention.
The additional activities and supplemental questions suggested in the article should help the students gain yet more practical skills and also stimulate intriguing questions, as well as offering sound answers.
Vladimir Petruševski, Republic of Macedonia









