What is chemiluminescence? Understand article
Glowing jellyfish, flickering fireflies, fun glow sticks; Emma Welsh introduces the beautiful and mysterious world of chemiluminescence.
Image courtesy of Erik
Solheim; image source:
Wikimedia Commons
Fireflies, jellyfish and glow sticks – one flies, one lives deep in the ocean and one provides entertainment in night clubs. What is the link? The answer is some intriguing chemical reactions that produce light.
Chemiluminescence is the production of light from a chemical reaction. Two chemicals react to form an excited (high-energy) intermediate, which breaks down releasing some of its energy as photons of light (see glossary for all terms in bold) to reach its ground state (see Figure 1, below).
A + B -> AB* -> Products + Light
Excited
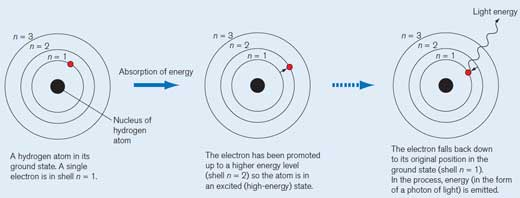
A hydrogen atom in its ground state. A single electron is in shell n = 1. Each shell has its own energy level.
When the hydrogen atom absorbs a quantum (defined amount) of energy, it is promoted to a higher energy level (shell n = 2) and is now in an excited (high-energy) state. We draw an asterisk (*) next to the molecule to indicate this.
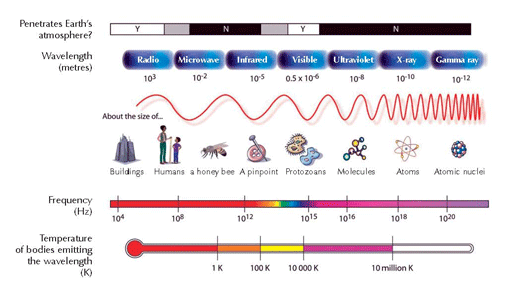
The electron falls back down to its original position in the ground state (shell n = 1). In the process, a packet of energy (a photon) is released in the form of electromagnetic radiation. The wavelength depends on the amount of energy. If the wavelength is within the range of visible light, the electron transition will be perceived as light of a particular colour. The wavelength determines the colour (see Figure 2, below)
Image courtesy of Chemistry Review
Chemiluminescent reactions do not usually release much heat, because energy is released as light instead. Luminol produces a light when it reacts with an oxidising agent; the chemistry of this reaction is shown in Box 1.
Box 1: Luminol, a glow-in-the-dark chemical
The release of a photon of light from a molecule of luminol is a fairly complex, multi-stage process. In a basic (alkaline) solution, luminol exists in equilibrium with its anion, which bears a charge of -2. The anion can exist in two forms (or tautomers), with the two negative charges delocalised on either the oxygens (the enol-form) or on the nitrogens (the ketol-form; see Figure 3, below).
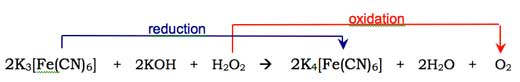
Molecular oxygen (O2) combines with the enol-form of the luminol anion, oxidising it to a cyclic peroxide. The required oxygen is produced in a redox reaction (i.e. one in which both reduction and oxidation occur) involving hydrogen peroxide (H2O2), potassium hydroxide and (for example) potassium hexacyanoferrate(III) (K3[Fe(CN)6], also known as potassium ferricyanide). The hexacyanoferrate(III) ion ([Fe(CN)6]3-) is reduced to the hexacyanoferrate(II) ion ([Fe(CN)6]4-, giving potassium ferrocyanide, K4[Fe(CN)6]), while the two oxygen atoms from the hydrogen peroxide are oxidised from oxidation state -1 to 0:
The cyclic peroxide then decomposes to give 3-aminophthalate (3-amino-1,2-benzenedicarboxylic acid) in an excited state, along with a molecule of nitrogen (N2) – see Figure 3, below. This decomposition reaction is favoured because the cyclic peroxide molecule is highly unstable, and the reaction involves breaking some weak bonds. It is also favoured because of the increase in entropy (disorder) due to the liberation of a gas molecule. When the excited 3-aminophthalate drops down to the ground state, a photon of blue light is released.
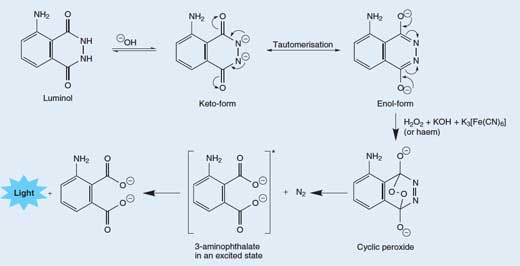
Tautomers are molecules with the same molecular formula, but different arrangements of atoms or bonds. The two tautomers can be interconverted; the curly arrows show the movement of electrons that brings about the change between the two forms. Click to enlarge image
Image courtesy of Chemistry Review
Chemiluminescence in forensics
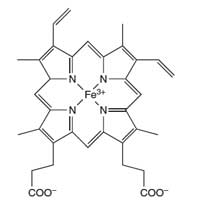
haemoglobin
The iron atom (Fe) in the
centre of the porphyrin ring
catalyses the reaction of
luminol
Image courtesy of Chemistry
Review
Forensic scientists use the reaction of luminol to detect blood at crime scenes. A mixture of luminol in a dilute solution of hydrogen peroxide is sprayed onto the area where the forensic scientists suspect that there is blood. The iron present in the haem unit of haemoglobin (see Figure 4) in the blood acts as a catalyst in the reaction described in Box 1. The room must be dark and if blood is present, a blue glow, lasting for about 30 seconds, will be observed. The forensic investigators can record this glow by using photographic film, which can be used as evidence in court for the presence of blood at the scene. (For a teaching activity about forensic science, see Wallace-Müller, 2011.
Because the iron acts as a catalyst, it is only required in trace amounts, therefore only a tiny amount of blood is required to produce a positive result. This means that blood can be detected even when it is not visible to the naked eye.

of a crime
Image courtesy of How Stuff
Works
One of the drawbacks of using luminol is that the reaction can be catalysed by other chemicals that may be present at the crime scene, for example, copper-containing alloys, some cleaning fluids such as bleach, and even horseradish. Clever criminals can clean up the blood with bleach, which destroys the evidence of the blood, but bleaching the carpet may alert people to the crime sooner. Urine also contains small amounts of blood, which can be enough to catalyse the reaction of luminol. Once luminol has been applied to the area, it may prevent other tests from being performed there. However, despite these drawbacks, luminol is still used by forensic scientists as a tool to solve crime.
In the nightclub
works. Click to enlarge image
Image courtesy of Chemistry
Review
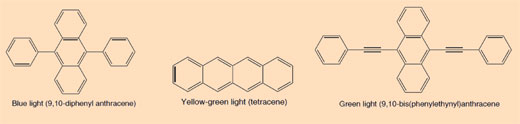
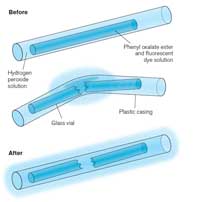
When you snap a glow stick and it begins to glow, the light produced is an example of chemiluminescence (see Figure 5). Glow sticks comprise a plastic tube containing a mixture including diphenyl oxalate and a dye (which gives the glow stick its colour). Inside the plastic tube is a smaller glass tube containing hydrogen peroxide. When the outer plastic tube is bent, the inner glass tube snaps, releasing the hydrogen peroxide and starting a chemical reaction that produces light (see Box 2). The colour of light that a glow stick produces is determined by the dye used (see Box 3).
Chemiluminescence reactions, such as those in glow sticks, are temperature-dependent. The reaction speeds up as the temperature rises – snapping your glow stick in hot water will produce a fantastic glow, but it will not last as long as it would at room temperature. Conversely, the reaction rate slows down at low temperature; this is why keeping your glow stick in the freezer for several hours can allow the stick to glow brightly again when it is removed and warmed up, long after it would otherwise have stopped glowing. The reaction does not stop completely in the freezer, but it does slow down so that the glow is barely detectable.
Box 2: Chemistry of glow sticks
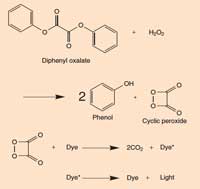
Image courtesy of Chemistry
Review
When diphenyl oxalate reacts with hydrogen peroxide (H2O2), it is oxidised to give phenol and a cyclic peroxide. The peroxide reacts with a molecule of dye to give two molecules of carbon dioxide (CO2) and in the process, an electron in the dye molecule is promoted to an excited state. When the excited (high-energy) dye molecule returns to its ground state, a photon of light is released. The reaction is pH-dependent. When the solution is slightly alkaline, the reaction produces a brighter light.
Safety note
Phenol is toxic, so if your glow stick leaks, take care not to get the liquid on your hands; if you do, wash them with soapy water straight away. See also the Science in School general safety note.
Box 3: What makes glow sticks different colours?
The dyes used in glow sticks are conjugated aromatic compounds (arenes). The degree of conjugation is reflected in the different colour of the light emitted when an electron drops down from the excited state to the ground state.
Living glow sticks

Image courtesy of Terry Priest;
image source: Flickr
Have you ever walked along a beach at night and seen sparks of light around your feet? Or been in the countryside at night and seen fireflies flitting about? These are examples of bioluminescence and around 90% of deep-sea life also exhibits this strange phenomenon. These organisms have evolved to produce light because it has many useful functions. Glowing can be used as a lure to catch prey, as camouflage or to attract potential mates. Some bacteria even use bioluminescence to communicate.
The term ‘glow worm’ describes the larvae of several species of insect, including fireflies; some of them glow to scare off predators, whereas other species use their glow to attract prey. There are species of squid and crustacean that can release clouds of bioluminescent liquid to confuse predators while they make their escape. Creatures living deep in the ocean have evolved to produce mainly blue or green light because it transmits well through seawater. This is because blue light has a shorter wavelength than red light, which means it is absorbed less readily by particles in the water.
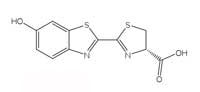
firefly luciferin.
Click to enlarge image
Image courtesy of Chemistry
Review (structure)
Bioluminescent reactions use ATP (adenosine triphosphate) as a source of energy. The structure of the light-producing molecules varies from species to species, but they are all given the generic name luciferin. The structure of firefly luciferin is shown in Figure 6, left. When fireflies glow, the luciferin is oxidised to produce an excited complex, which falls back down to the ground state, releasing a photon of light, just like the chemiluminescent reaction of luminol described in Box 1. However, fireflies do not use hydrogen peroxide and potassium hexacyanoferrate(III) to oxidise luciferin; instead they use molecular oxygen and an enzyme called luciferase (this is also a generic name – luciferases vary from species to species).

discovered in the jellyfish
Aequorea victoria
Image courtesy of Typoform /
the Royal Swedish Academy of
Sciences (RSAS)
Luciferase
Luciferin + O2 → Oxyluciferin + Light
There have been a number of experiments investigating aequorin, a protein found in certain jellyfish, which produces blue light in the presence of calcium (see Shaw, 2002, and Furtado, 2009) and can thus be used in molecular biology to measure calcium levels in cells. Some scientists have come up with other ideas for utilising bioluminescence in the future, for example self-illuminated Christmas trees. Can you think of any other exciting potential uses for this amazing natural phenomenon?
Glossary
Anion: an atom (or group of atoms) that bears a negative charge.
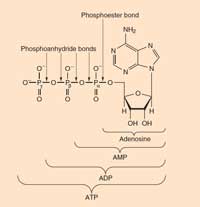
Image courtesy of Chemistry
Review
ATP: adenosine triphosphate occurs in all known life forms. It is the primary energy currency in cells. ATP is formed from ADP (adenosine diphosphate) and phosphate during energy-yielding reactions (such as the oxidation of glucose), and is broken down (to ADP and phosphate) to release this energy in order to drive unfavourable reactions.
Bioluminescence: The production of light by living organisms. Bioluminescence may result from the absorption of light (fluorescence or phosphorescence, e.g. in many deep-sea fish) or from a chemical reaction (chemiluminescence, e.g. in fireflies).
Catalyst: A substance that makes a reaction occur faster, but that does not undergo a permanent chemical change during the reaction (i.e. is not used up in the reaction). Catalysts work by providing an alternative route for the reaction that is lower in energy.
Chemiluminescence: A type of luminescence in which the electrons are excited by a chemical reaction, for example the reaction of luminol described in Box 1.
Conjugated: Conjugated systems mainly arise in chemistry when there are double bonds next to each other. The atoms in a conjugated system are held together by covalent bonds and have alternating single and multiple bonds (mainly double bonds, but triple bonds are also capable of being in conjugation). Alkenes are flat; conjugated systems must always be planar to allow delocalisation of the electrons throughout the system. The dye molecules in Box 3 are all examples of conjugated compounds.
Covalent bonds: Bonds between two atoms where a pair of electrons are shared between them.

delocalised in a conjugated
system
Image courtesy of Chemistry
Review
Delocalised: When molecules have conjugated bonds, the electrons are free to move around throughout the entire conjugated system. These are referred to as delocalised electrons. The electrons in a benzene ring are delocalised, and this is why all the carbon-carbon bonds are the same length.
Fluorescence: A type of luminescence in which the electrons are excited by light, e.g. in the security markings on banknotes.
Luminescence: The production of light, usually at low temperatures, for example by chemical reactions or electrical energy. Incandescence, in contrast, is light generated by high temperatures.
Phosphorescence: As fluorescence, but the glow lasts for longer (according to some definitions, over 10 nanoseconds), for example glow-in-the-dark stickers.
Acknowledgement
The original version of this article was published in Chemistry Review and is reproduced with kind permission by the publisher, Philip Allan. To subscribe to Chemistry Review, a journal aimed at school chemistry students aged 16-19, visit: www.philipallan.co.uk/chemistryreview
References
- Furtado S (2009) Painting life green: GFP. Science in School 12: 19-23.
- Shaw A (2002) Genetic chess by the light of a jellyfish. Chemistry Review 12(1): 2-5
- Wallace-Müller K (2011) The DNA detective game. Science in School 19: 30-35.
Resources
- For some experiments with luminol, see Declan Fleming’s website for older school students, all about the chemiluminescence of luminol: www.chm.bris.ac.uk/webprojects2002/fleming/experimental.htm
- To learn about other types of light in chemistry, see:
- Douglas P, Garley M (2010) Chemistry and light. Science in School 14: 63-68.
Review
This article offers a way to motivate students to understand chemical reactions. Even if they are not keen to know why a glow stick glows in the dark, they will surely be eager to find out how fireflies or jellyfish produce light, or to discover how blood is detected at crime scenes. The article can serve either as an introduction to chemical reactions or to give attractive examples of redox reactions and also to illustrate the levels of energy in the shell of an atom.
The article can be adapted for different age ranges and for different subjects and topics. For students aged 14-15, it could be used to teach chemistry (atomic structure and movement of electrons between shells, introduction to chemical reactions) or biology (bioluminescence). For this age group, the teacher would need to simplify the information in the article and omit the details of the reactions. For students aged 16-18, the article could be used to teach chemistry (redox reactions, catalysts, the influence of temperature on reaction speed, the effect of pH on a reaction, and covalent bonds), physics (the electromagnetic spectrum and photons) or genetics (genetic engineering). Suitable comprehension questions include:
- What is chemiluminescence?
- What do forensic scientists use chemiluminescence for?
- Explain some biological functions of bioluminescence.
- Why should you keep your glow stick in the freezer when you are not using it?
- How could you make a self-illuminated Christmas tree?
Ana Gil, Spain