X-rays shed light on enhancing zinc uptake in pepper plants Understand article
The following was adapted from an ESRF News article.
Zinc is an important trace element for plants and animals alike. Learn how nanoparticles could supply zinc to crops without having to add it to the soil.
Why is zinc important?
Zinc (Zn) is a vital element for animals, including humans. It is essential for growth and health and we get it from our diet. But where does the zinc in food come from? Plants also require zinc to grow, and since they don’t eat, they must get it from the soil. In farming, zinc-deficient soil significantly impacts crop quality, causing yellowing of the leaves, stunted growth, and small or deformed leaves. This means that it is often necessary to use zinc fertilizers. However, conventional methods of adding zinc to the soil result in low zinc uptake by the plants, and increase the risk of soil and groundwater pollution.[1]

Image: Alandmanson/Wikimedia Commons, CC BY-SA 4.0
Could nanoparticles be part of the solution?
To address these issues, researchers are exploring the application of zinc-based nanoparticles directly to plant leaves (foliar application) for controlled, sustained zinc delivery without having to add zinc to the soil. This approach has potential, but the mechanisms underlying the uptake of zinc nanoparticles into the leaves (foliar uptake), their transport within the plant, and their transformation along the way are not fully understood. Which routes lead to the foliar uptake of these nanoparticles? In which compartment of the leaf do they accumulate and transform? Do they reach the vasculature and spread to the developing tissues that need zinc the most? In particular, the influence of nanoparticle surface modifications on these processes required further investigation to ensure the best possible uptake.[2]
How can we study this?
An international team of researchers studied the bioavailability of different zinc nanoparticles after they were applied to the leaves of bell pepper (capsicum) plants.[4]

Image: Léna/Wikimedia Commons, CC BY 3.0
The pepper plants were exposed to two types of nanoparticles: bare zinc oxide nanoparticles and zinc oxide nanoparticles coated with a zinc-phosphate shell. They then explored how the nanoparticles interacted with the leaves, from their deposition on the cuticle to their uptake in the interior of the leaf (the epidermis), the mesophyll where most of the photosynthesis takes place, and the vasculature. They also tracked zinc transport from leaf to stem and fruits.
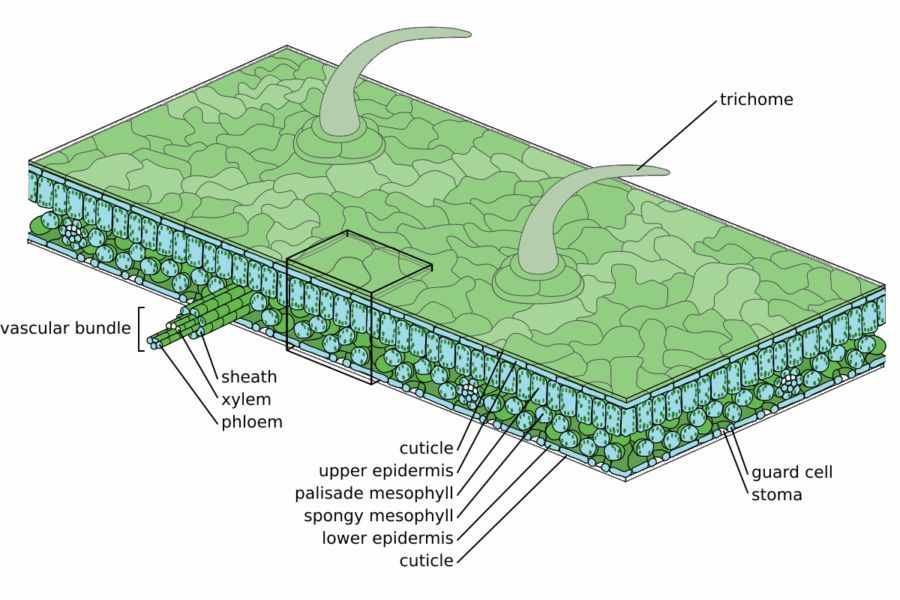
Image: Zephyris/Wikimedia Commons, CC BY-SA 3.0
To track the fate of the zinc nanoparticles in the plants, leaf and stem samples were collected two hours and one week after leaf application. Small discs were punched out of the leaves (figure 4 b), then the samples were placed in a special resin and flash frozen in liquid nitrogen to preserve them (figure 4 c). Very thin cross-sections just 20 µm thick (finer than a human hair) were then cut using a precision cutting machine called a microtome.
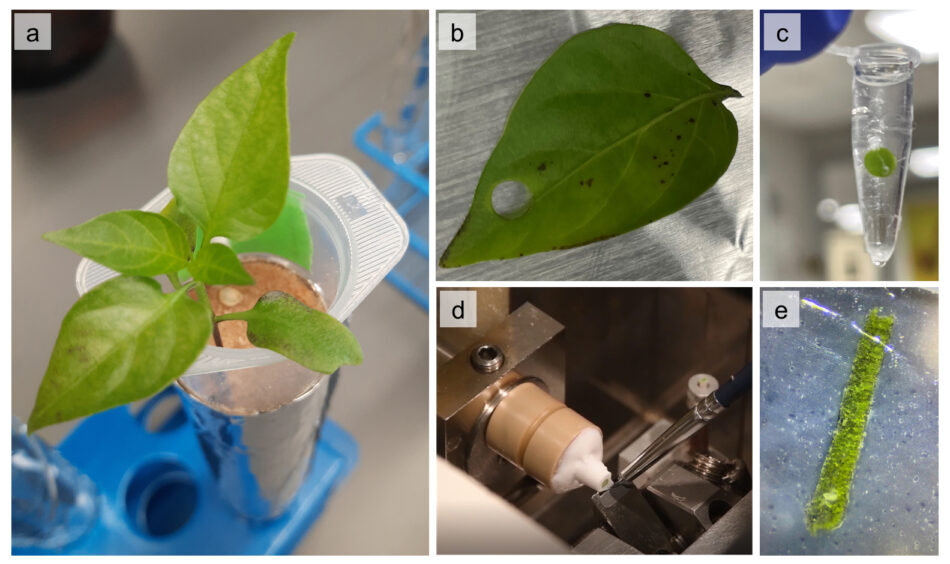
Image from ESRF News
These thin samples were then analyzed at the European Synchrotron Radiation Facility (ESRF) in Grenoble to answer two questions: how does the zinc distribute in the plant tissues, and what chemical form does the zinc take (native nanoparticulate form or transformed into other zinc species)? The researchers used two special X-ray techniques: micro X-ray fluorescence (μ-XRF) and micro X-ray absorption near-edge structure (μ-XANES) spectroscopy. μ-XRF collects all the fluorescence signals of each pixel, enabling researchers to construct a map of the elements at each position in the sample. μ-XANES spectroscopy gives information on the atoms surrounding the zinc, which indicates its chemical form. The beamline at ESRF, at which this was done, allows these measurements to be performed with a very small beam size (ca. 500 nm in diameter), which is required to study these very small structures.
The ESRF

The ESRF is the world’s brightest synchrotron light source. Every year, 9000 scientists from 20 partner countries and from around the world travel to Grenoble to use its extremely brilliant X-rays for leading-edge research. This fundamental and applied research aims to address the complex global challenges that our society faces, such as health, energy, and the environment. It also contributes to the development of new technologies for industry and to preserving humanity’s cultural heritage, lighting the way to a sustainable and peaceful future.
Does nanoparticle structure make a difference?
The results revealed clear differences in what happened to the zinc particles in pepper plants and where they ended up depending on how the nanoparticles were designed.
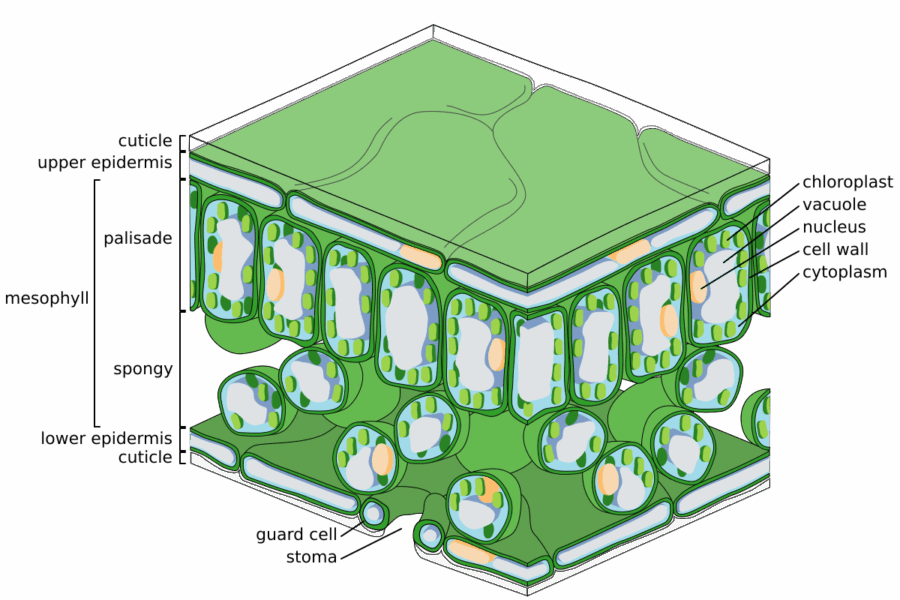
Image: Zephyris/Wikimedia Commons, CC BY-SA 3.0
The bare zinc oxide nanoparticles resulted in high zinc accumulation in the lower epidermis cell walls and cytosol of leaves (figure 6), as well as in the stem epidermis one week after exposure. In contrast, plants treated with the nanoparticle that had a zinc phosphate shell showed more zinc accumulation in leaf and stem vasculature, suggesting that uptake in the vasculature was improved for this treatment. These findings indicate that the surface chemistry of the nanoparticles may cause differences in how zinc is distributed, stored, or transformed within the leaves of pepper plants, and offers ideas to make zinc delivery via leaves more efficient.
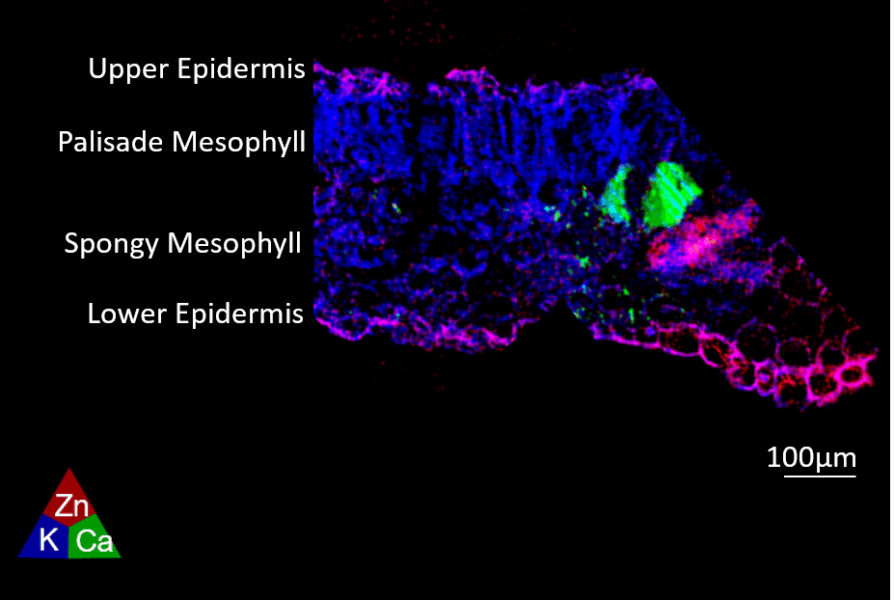
Image adapted from Ref. [4]
The μ-XANES spectroscopy, which was done for different points of interest on each sample, revealed more details of how the plants was using zinc. Firstly, none of the zinc taken up inside the leaf was detected in nanoparticle form, even just 2 h after application, which indicates that the nanoparticles dissolved very fast upon uptake. Secondly, after 2 h of exposure to the uncoated zinc nanoparticles, the zinc was mainly associated with carboxyl and phosphate groups, which indicates that the zinc was mainly associated with cell walls (which are rich in peptides containing carboxyl groups) and/or precipitated inside the cytosol of the cells right after uptake. After treatment with the zinc phosphate coated nanoparticles, more points on the leaf showed zinc associated with thiol groups. This indicates it was binding to metalloproteins,[3] which are proteins that bind metal ions, often via thiol groups on the amino acid cysteine. The fact that the zinc from the zinc phosphate coated nanoparticles was taken up into these proteins suggests better incorporation into the plant compared with zinc from the bare zinc oxide nanoparticles.
Where do we go from here?
Altogether, these results indicate that zinc from zinc phosphate coated nanoparticles moved more easily within the plant than the zinc from the bare zinc oxide nanoparticles. The presence or absence of phosphate on the surface of the nanoparticles caused differences in both translocations inside the plant and cellular internalization of zinc after uptake. This change in mobility due to the phosphate shell correlated with the fact that zinc was only transported into the pepper fruits after treatment with the zinc phosphate coated nanoparticles. This is of interest since there are two possible goals to supplementing crops with zinc: 1) improving the health of the plants to improve crop yields and 2) increasing the amount of zinc in the part of the plant we eat to improve human nutrition. It’s important to note that, at this stage, the zinc was no longer in the nanoparticle form but had been incorporated into the plant tissues, so the nanoparticles didn’t end up in the fruit.
Image: Wessels et al./Our World in Data, CC BY 4.0
Overall, the results showed that the zinc phosphate coating on zinc oxide nanoparticles seems to boost zinc uptake after application and accelerate phloem loading for distribution around the plant. This study highlights how small differences in the structure of a nanoparticle can have big effects on their fate in living things. This effect is well known in medicine, but other applications such as agriculture are sometimes less highlighted, although agricultural practices have a huge effect on both human and planetary health with links to sustainable development goals (SDGs) 2 (zero hunger), 3 (good health and well-being), 6 (clean water and sanitation), 12 (responsible consumption and production), 13 (climate action), 14 (life below water), and 15 (life on land). Research like this could be key for enhancing nutrient delivery in plants and bolstering crop resilience. Further research into the applications of phosphate-coated nanoparticles and their effects on plant nutrient uptake mechanisms are needed to harness their potential benefits in agriculture.
Glossary
Bioavailability: how easily a nutrient or other molecule in a particular form can be absorbed and used by the target organism. For example, the heme iron found in animal products has good bioavailability for humans but pure iron metal doesn’t; swallowing a lump of iron (not recommended!) won’t help much with iron deficiency.[6]
Crops: plants grown for human use, often for food
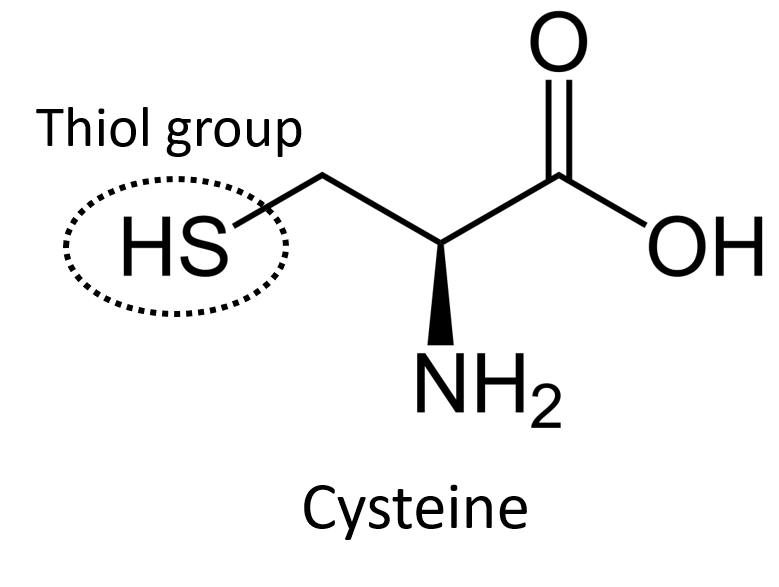
Cysteine: an amino acid that contains a thiol group, which is used in proteins to build disulfide bridges or to bind to metal ions through coordination chemistry
Fertilizer: a substance (natural or synthetic) that applied to the soil to provide plants with the essential nutrients they need to grow. Many fertilizers can cause environmental problems if they leach into the environment.
Nanoparticles: really small particles less than 100 nm in diameter
Metalloproteins: proteins that bind to metal ions as part of their function. They have a wide range of important biological functions, including oxygen transport and enzyme catalysis.
Synchrotron: a circular particle accelerator. The synchrotron at ESRF is used to produce extremely bright X-ray beams (billions of times brighter than medical X-rays) for research.
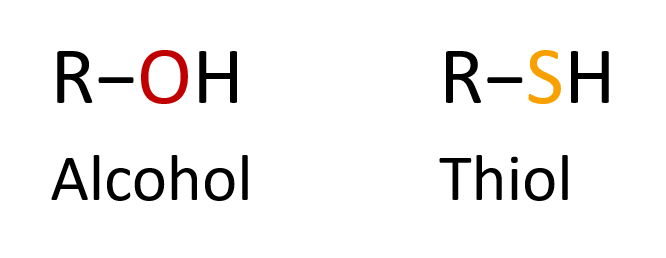
Thiol group: a sulfur-containing functional group that is like the alcohol functional group (hydroxyl group) but with sulfur instead of oxygen
Vasculature: in plants, the xylem and phloem vessels that transport water and nutrients around the plant
Zinc oxide: a zinc compound that is often used in zinc supplements (for humans) and zinc fertilizers. It is also used in so-called ‘physical sunscreens’.
Zinc: a transition metal that is an essential element for both plants and animals
Acknowledgements
Part of the research that led to the scientific publication of this work was funded by the French National Centre for Scientific Research (CNRS), and the European Research Council (ERC StG, LEAPHY 101041729).
References
[1] Martins NCT et al. (2020) Composites of Biopolymers and ZnO NPs for Controlled Release of Zinc in Agricultural Soils and Timed Delivery for Maize. ACS Applied Nano Materials 3: 2134-2148. doi: 10.1021/acsanm.9b01492
[2] Avellan A et al. (2021) Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation Environ. Sci. Technol. 55: 13417-13431. doi: 10.1021/acs.est.1c00178
[3] Hamzah Saleem M et al. (2022) Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Frontiers Plant Sci. 13: 1033092. doi: 10.3389/fpls.2022.1033092
[4] Rodrigues S et al. (2024) Effect of a Zinc Phosphate Shell on the Uptake and Translocation of Foliarly Applied ZnO Nanoparticles in Pepper Plants (Capsicum annuum). Environ. Sci. Technol. 58: 3213–3223. doi: 10.1021/acs.est.3c08723
[5] Wessells KR, Brown KH (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PloS one, 7: 50568. doi: https://doi.org/10.1371/journal.pone.0050568
[6] Hurrell R et al. (2010) Revised Recommendations for Iron Fortification of Wheat Flour and an Evaluation of the Expected Impact of Current National Wheat Flour Fortification Programs. Food and Nutrition Bulletin 31: 7−21. doi:10.1177/15648265100311S102
Resources
- Learn why zinc is an essential mineral for humans and how we get it from our diet.
- Explore global data on zinc deficiency on an interactive map.
- Learn why zinc is important for plants.
- Read about some of the problems of excess fertilizer use, especially nitrogen fertilizers.
- Learn why excess zinc in the environment can cause problems.
- Read about the vital importance of sustainable nutrient management.
- Read about how all sunscreens are made of chemicals and how physical sunscreens work in a very similar way to chemical sunscreens.
- Watch a video on the myths and misconceptions surrounding so-called ‘physical sunscreens’.
- Analyze coffee grounds to determine their suitability as a soil enhancer, while learning about the concepts of the circular economy: Messori G (2025) Chemistry in a coffee cup: does coffee waste contain key elements for plant growth?. Science in School 73.
- Discover how bacteria from ants’ feet could help us reduce pesticide use: Jensen IC (2024) Footprints in the agar: growing bacteria from ants’ feet to combat plant diseases. Science in School 67.
- Be amazed by the sheer number of particle accelerators around the world and the various ways in which they are used: Lewis J, Darve C (2024) Accelerators are everywhere, perhaps closer than you think…. Science in School 69.
- Investigate how much herbaceous plants contribute to biodiversity and CO₂ sequestration: Barbato R (2024) Biodiversity and biomass in the school garden. Science in School 69.
- Explore the challenges of genome sequencing: Dyer S, Jackson B (2024) Plant genetics: explore DNA extraction and the challenges of gene sequencing. Science in School 68.
- Learn how to make statistical evaluations to measure plant growth: Brown J, Karamurzina S, Zharylgasin S (2020) Grow your own statistical data. Science in School 50.
- Investigate the factors affecting plant growth and devise a plan for growing plants on the Moon: Hardie K, Cardoso C (2020) Astrofarmer: how to grow plants in space. Science in School 49: 33–37.
- Learn how plants defend themselves against pathogens: Harant A, Pai H, Cerfonteyn M (2023) Plant pathology: plants can get sick too! Science in School 62.
- Read about the first land plants and how they changed our world: Streubel S (2023) When plants moved ashore and changed the planet. Science in School 64.
- Learn how scientists are studying pea-protein foams in order to develop better plant-based food alternatives: Matsarskaia O (2025) Pea-based foams for a greener cappuccino. Science in School 72.
- Discover how trees use chemicals to communicate with soil microbes: Rumeau M (2024) Exploring the dialogue between trees and soil microbes. Science in School 68.
- Explore how the unique characteristics of seagrasses are vital for the health of our planet: Crouch F (2024) Seagrass the wonder plant! Science in School 67.





