
Build your own virtual accelerator
Build your own virtual particle accelerator with the aid of the acceleratAR app and gain a hands-on, immersive understanding of how these machines…

The following was adapted from an ILL News article
Dirty windows can harbour potentially harmful pollutants under protective films of fatty acids from cooking emissions – and these can hang around for long periods of time.
Fatty acids are the most abundant molecules in animal and vegetable oils. These molecules are composed of a polar region, which is attracted to polar surfaces like glass, and a non-polar region. This makes them surface-active and also means that they are prone to self-assemble to maximize their polar/polar and non-polar/non-polar contacts, similar to the phospholipid molecules in our cell membranes. The result is that when these molecules are released into the air, for example, when fats are heated during cooking, and hit a solid polar surface, like a window, they form a self-organised thin film that builds up over time. According to a new study led by researchers at the University of Birmingham, UK, the fatty acids in cooking emissions are highly stable and not easily broken down in the atmosphere.[1]
Compounds on air-exposed surfaces are often broken down through reactions with atmospheric molecules like ozone or water. To investigate how this applies to fatty acid films from cooking emissions, the thin fatty acid films were exposed to ozone and humidity to simulate atmospheric ageing. The results showed that the films are only very slowly broken down in the atmosphere. The main change in the film was moisture uptake and an increase in roughness due to uneven water uptake and oxidation. This means that these films could act as a protective layer, burying potentially harmful pollutants that also often deposit on window surfaces. Senior author Dr Christian Pfrang said, “The fatty acids in these films are not, by themselves, particularly harmful – but because they are not being broken down, they are effectively protecting any other pollutants that might be trapped underneath.”
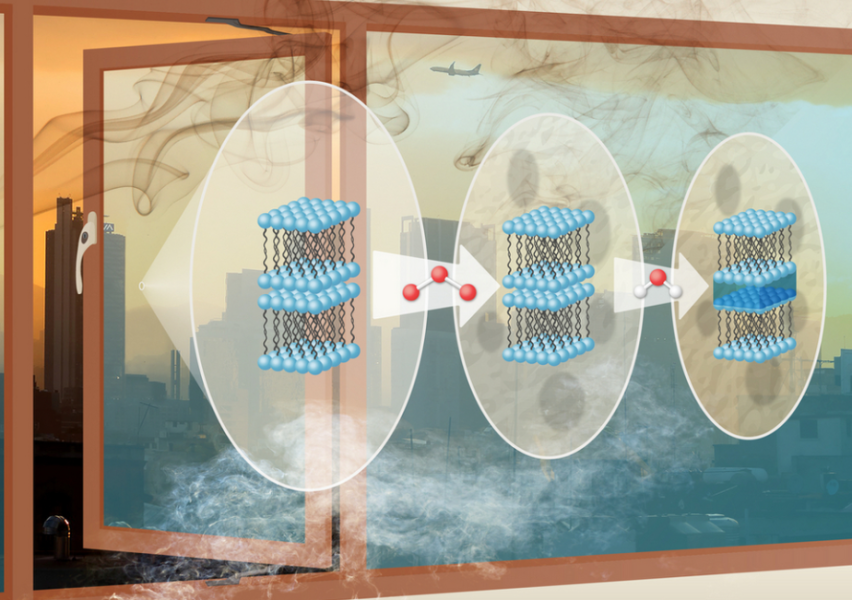
This complex study was published in the journal Environmental Science: Atmospheres and was carried out in partnership with experts from the University of Bath, Diamond Light Source, and ISIS Neutron and Muon Source in the UK, and the Institut Laue-Langevin (ILL) in France. The team worked on laboratory ‘proxies’ – samples of material engineered in the lab to approximate real-world samples. These were spun into super-thin films of fatty acids just a few tens of nanometres in thickness.
The Institut Laue-Langevin (ILL) is an international research centre at the leading edge of neutron science and technology located in Grenoble, France. As a service institute, the ILL makes its facilities and expertise available to visiting scientists. Every year, about 1400 researchers from over 40 countries perform research at the ILL in a range of fields: materials science, biology, medicine, physics, chemistry, and environmental science.

Neutrons produced by the ILL nuclear reactor are a powerful probe to study small samples of materials. They have specific properties that enable them to yield information that is often impossible to obtain using other techniques. Neutrons interact with the nuclei of matter, and observing how they are deflected and change speed gives precise information about the position and motion of the nuclei. They also behave like small compass needles and can provide unique insight into magnetism.
At the ILL, neutrons are produced in a high-flux nuclear reactor from the fission of a highly enriched U235 fuel element, similar to commercial power plants. However, whereas in electric power plants the decay process is exploited to produce energy, at the ILL the reactor is used to extract neutrons for scientific research.
The researchers used both neutrons and X-rays to study the nanoscale composition of the films and the changes in their surface structures. By changing the humidity and amount of ozone – a key pollutant involved in atmospheric ageing – the researchers were also able to mimic the behaviour of the films over time. Neutrons played a key role in this study due to their high sensitivity to the water within the moisture-swollen films. Thanks to the high-resolution detector on the FIGARO instrument at ILL, the order of the fatty acids and their position in the layer could be observed.
The researchers found a self-organised arrangement of repeating molecular sheets within the films – a so-called lamellar phase. This arrangement makes it difficult for smaller molecules, like ozone, to access the reactive parts of the fatty acids to oxidize them and thus remove them from the window. After the film was deposited and exposed to ozone, the only change was that the surfaces of the films became less smooth, most likely due to the penetration of ozone through defects in the film. This makes the film more likely to take up water from the atmosphere, which can contain toxic aerosols.
This research suggests that it could be beneficial to reduce the accumulation of cooking emissions on surfaces by either regular cleaning or reducing polar surfaces in kitchen environments. However, more research is needed to investigate mixtures of molecules that more closely resemble real cooking emissions.
[1] Pfrang C et al. (2022) The evolution of surface structure during simulated atmospheric ageing of nano-scale coatings of an organic surfactant aerosol proxy. Environmental Science: Atmosphere 2: 964–977. doi: 10.1039/D2EA00011C.

Build your own virtual particle accelerator with the aid of the acceleratAR app and gain a hands-on, immersive understanding of how these machines…

The asteroid that killed the dinosaurs struck Earth during springtime. Scientists have determined this by analyzing the remains of fish that died…

Basic research is often misunderstood by the public and misconstrued by the media. Try this role play to learn how research is funded and how basic…