Supporting materials
Download
Download this article as a PDF

Helium: gas of awe, wonder, and worry. Is it time to give this noble gas the respect it deserves?

Helium: such an inspiring gas to meet. The second most common element in the universe, the stuff of stars, is less dense than air, so if you lose your grip on a helium balloon, it rises into the air. Breathing a mouthful of helium can make you sound like Donald Duck, and the reason lies in vocal folds and the speed of sound in helium. However, don’t try this at home: the risk assessment includes potential death.[1]
What do we know about helium? Where do we get it from? What do we use it for? What of our future relationship with this unreactive and fascinating gas?
The word helium comes from Helios, Greek for the Sun, because this element was actually discovered in the Sun before it was known on Earth. The spectrum of the Sun’s corona, the ‘crown’ of hot gas surrounding the Sun’s glowing surface, was analysed during a solar eclipse in 1868.

Different elements absorb different colours of light, giving a ‘bar code’ for the different elements present.
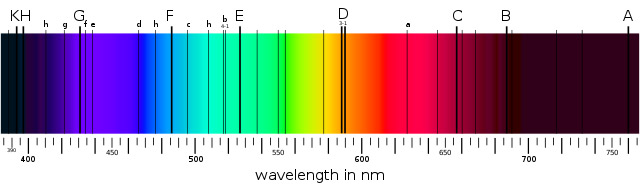
In addition to the bar codes for known elements like hydrogen and sodium, Jules Janssen from France found a set of lines that didn’t fit with any known element, and Norman Lockyer from England suggested that the Sun contained an element unknown on Earth and gave it the name helium.
In 1881 the same pattern was discovered in gas from Mount Vesuvius in Italy. Eventually, helium was collected: a very low-density, unreactive gas to join argon, xenon, and krypton, and in 1902, Mendeleev added a new column to his periodic table for these ‘noble gases’.
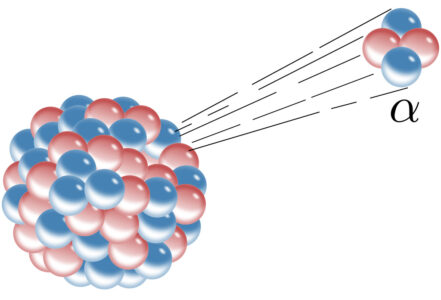
Helium is the second most common element in the Universe; it was formed during the Big Bang and there is lots in stars, which release energy by fusing hydrogen atoms to give helium. However, Earth’s helium is produced by alpha decay within the Earth’s crust. Radioactive elements, such as isotopes of uranium, emit alpha particles, which are helium nuclei. These doubly positively charged alpha particles pick up a couple of electrons and there you are: a helium atom.
The very small helium atoms find their way through gaps between particles in permeable rocks, so helium leaks out of the Earth over millions of years. There are just a few rare wells of helium gas, which form when pockets of helium are trapped by impermeable rock.
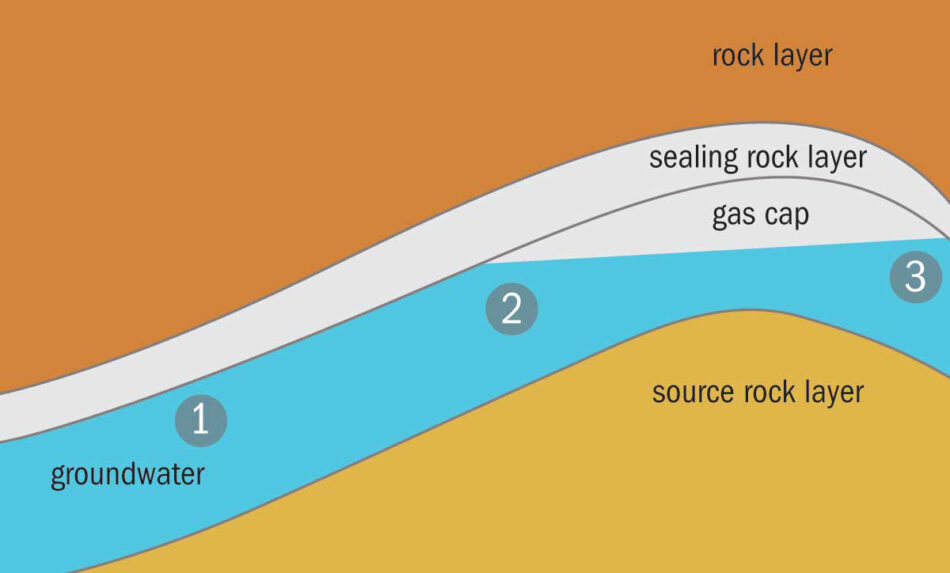
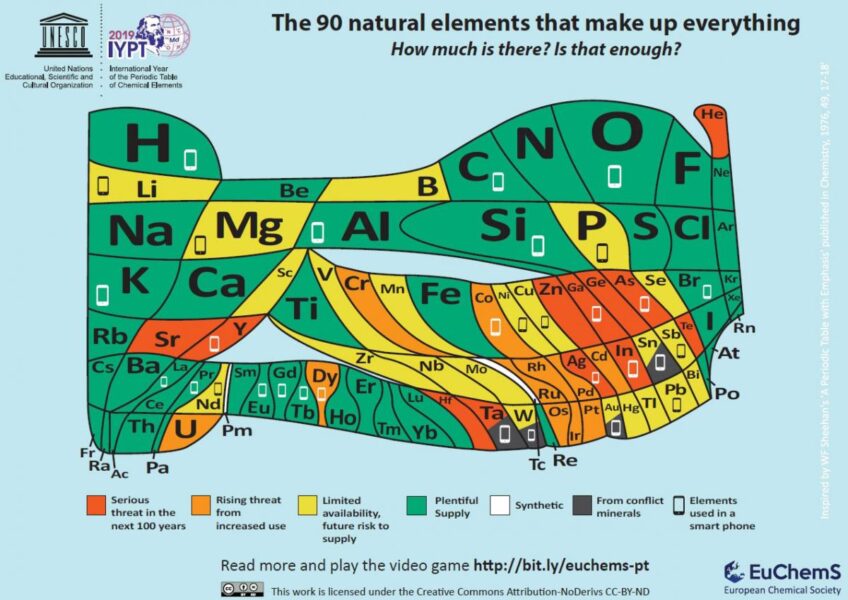
Examples can be found in Qatar, the USA, Russia, and Algeria. Some of these are becoming depleted, and the geopolitics of others makes supply unreliable. The largest well is estimated to about seven times the annual global consumption,[2] and the estimated total US known reserves might supply twenty years. However, helium is an element, so we can’t synthesise more of it, and we have used millions, or even billions, of years’ accumulation of this precious element in just a few decades.
Helium is unique amongst the light elements in that it is a product of the Earth, rather than the star debris that preceded it. All of Earth’s helium has been made during its own history.
Why have billions of years of helium release from rocks not given Earth a helium atmosphere? Helium atoms are low-mass, and so they are fast-moving particles in air. Any helium atoms high enough and fast enough can escape gravity’s pull: Earth leaks helium to space.
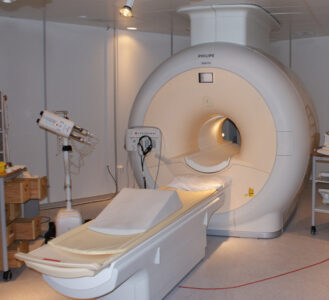
The biggest application of helium is in cryogenics. Liquid helium is a ‘magic ticket’ towards absolute zero – the lowest temperature possible. Helium condenses at -269°C (4 K), and at this temperature, most metals become superconductors and let an electric current flow with zero resistance. This means that helium is key wherever very strong electromagnets are needed.
An example is magnetic resonance imaging (MRI) scanners. They make details like torn ligaments, tumours, and heart defects visible without surgery, which has transformed medical diagnosis. They account for about 30–35% of the helium used each year globally,[3,4] but they remain rare in many parts of the world, so this demand should grow. Do you know anyone who has had an MRI scan, possibly a lifesaving one? This wouldn’t be possible without helium.
In scientific research, we know about the structures of proteins and other complex molecules thanks to nuclear magnetic resonance (NMR) spectrometers, the cousins of MRI. Particle accelerators such as those at CERN, the birthplace of the world-wide-web and the site of the hunt for the Higgs Boson, also depend on vast electromagnets cooled by helium to steer charged particles.
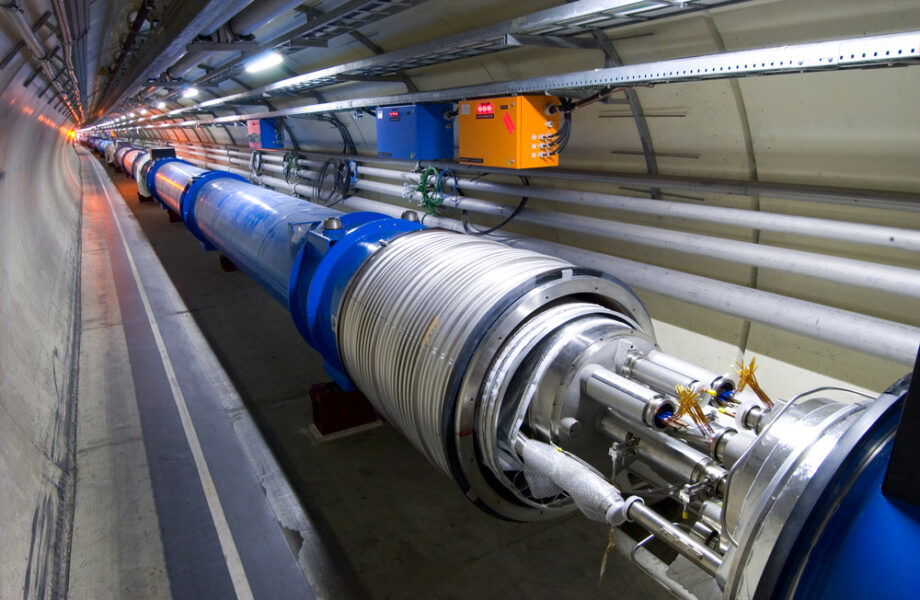
As a noble gas, helium is also inert, which makes it useful in applications where a non-reactive atmosphere is needed, for example, in certain kinds of welding and to purge production lines of other more reactive substances. Semiconductor manufacture for electronics relies on helium because of its inert nature and fluid properties.[5]
Helium’s inertness also means that it is non-toxic. Deep-sea divers use helium in their breathing mix to reduce the nitrogen absorbed into the blood during deep dives. Nitrogen dissolved in the blood can form bubbles when the diver surfaces, leading to ‘the bends’, which is painful and can be deadly.
Finally, the small size of helium atoms means that helium gas can find its way through gaps on the atomic scale, which makes it useful in research and industry to check for leaks.
So, we have a problem. Modern life depends on the use of an element that is not only finite but leaks away from the Earth, and whose usage is quickly outstripping reserves. Radioactive decay in the Earth is producing more as you read this article, but at a rate that is tiny compared to the rate of use.
Helium is a key element in our lives, but how common is this knowledge? Does it need a better press along with the scientific campaign[6] for its conservation, recycling, and reuse?
Meanwhile, 17% of our global consumption[4] is used to fill balloons. A precious and important gas is lost to space and the balloons fall as litter, endangering wildlife.[7] Should we rethink the ethics of this? Are party balloons really worth serious helium shortages for future generations?[8]
The author was inspired to write this article after attending an impassioned lecture by Professor David Cole-Hamilton, author of the Elements in Danger version of the periodic table.
[1] An article in The Times on the deaths caused by helium misuse in the UK: https://www.thetimes.co.uk/article/more-than-500-deaths-are-linked-to-helium-misuse-226h83spt.
[2] A news article on the discovery of a helium well: https://www.ox.ac.uk/news/2016-06-28-huge-helium-discovery-life-saving-find.
[3] Olafsdottir AH, Sverdrup HU (2020) Assessing the Past and Future Sustainability of Global Helium Resources, Extraction, Supply and Use, Using the Integrated Assessment Model WORLD7. Biophysical Economics and Sustainability 5:6. doi: 10.1007/s41247-020-00072-5.
[4] More details on the uses of helium in a table from Ref. [3].
[5] The uses of helium in electronics in this special feature by gasworld: https://www.linde-gas.com/en/images/Gasworld%20Helium%20in%20Electronics%20January%202016_tcm17-419249.pdf.
[6] A YouTube video to raise awareness of helium conservation: https://youtu.be/sHXu65zbpx8.
[7] An article by the Royal Society of Chemistry on helium and other elements in danger: https://www.rsc.org/news-events/opinions/2019/jan/elements-in-danger/.
[8] The website for the US campaign against the release of balloons and the misuse of helium: https://balloonsblow.org/.
Download this article as a PDF