Little wonder: microscale chemistry in the classroom
Good things come in small packages: discover how microscale experiments can have a big impact in STEM…

Learn how to do quantitative chemistry experiments involving reaction rates using microscale techniques that are relatively easy and quick to set up, without expensive equipment.
Quantitative chemistry allows students to generate experimental data and link it with their knowledge of particles, chemical equations, and numerical equations. This can be difficult for students to master and requires practice and familiarization with the equations and formulae involved.
Microscale chemistry methods use less reagents, reduce costs, decrease the time taken for preparation, and minimize the production of hazardous substances.[1] These microscale practical activities are relatively simple and quick to do, and thus, can help students focus on the chemistry and reduce the load on working memory. Despite the small masses and volumes involved, the data generated from microscale experiments shows equivalent or better results than traditional equipment, although a comparison of techniques is a useful exercise in error analysis.
Rates of reaction lessons can bring together concepts about how reactions are proceeding and how the particles are interacting. This can lead into collision theory, which identifies the factors required for a successful collision (sufficient energy and correct orientation of the particles) and factors that affect the rate of reaction, such as concentration, temperature, surface area, and the presence of catalysts.[2]
These activities can be done with students aged 16–18 and can take about 50–100 minutes each, depending on the number of repeats performed.
The reaction between sodium thiosulfate and hydrochloric acid produces a fine precipitate of solid sulfur. This allows the relative rate of reaction to be calculated by measuring the time needed for the reaction mixture to become opaque.
This microscale method has advantages over the large-scale reaction. Smaller amounts of chemicals are used, thereby reducing cost and preparation time. Due to the reduced volumes used, the total volume of toxic sulfur dioxide produced is significantly reduced, lowering the exposure to students and teachers. A stop bath is included, which stops the reaction by neutralizing hydrochloric acid and acidic sulfur dioxide, preventing further release of sulfur dioxide.
The time taken to do the experiment is reduced and repeats can be done if desired.
Chemicals
1 M hydrochloric acid is an irritant and the solution of phenolphthalein in ethanol is highly flammable. Use eye protection and do not perform the experiments near any sources of ignition.
Disposal
Pour the completed reaction mixtures into the prepared alkaline stop baths. If the waste mixture loses the pink colour, add additional 0.5 M sodium carbonate solution. Pour the stop-bath mixture down the foul-water drain with additional tap water. Rinsed glassware can be washed up as normal.
| Reaction number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1 M hydrochloric acid (cm3) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Water (cm3) | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 |
| 0.1 M sodium thiosulfate solution (cm3) | 4.5 | 4.0 | 3.5 | 3.0 | 2.5 | 2.0 | 1.5 | 1.0 |
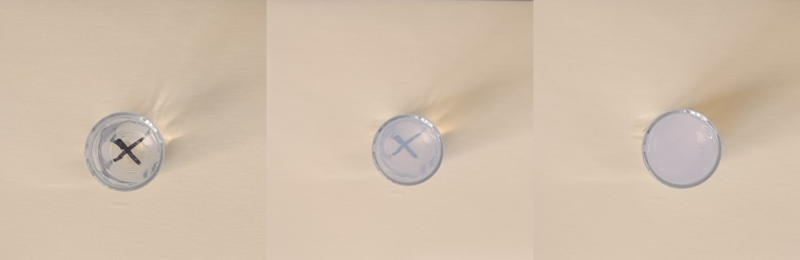
A sample table of results obtained from this experiment is given below.
| Volume of sodium thiosulfate (cm3) | Time taken for cross to disappear (s) | Relative rate of reaction, 1/t (s−1) |
| 1.0 | 183 | 0.0054 |
| 1.5 | 143 | 0.007 |
| 2.0 | 111 | 0.009 |
| 2.5 | 82 | 0.015 |
| 3.0 | 63 | 0.016 |
| 3.5 | 58 | 0.017 |
| 4.0 | 47 | 0.021 |
| 4.0 | 43 | 0.023 |
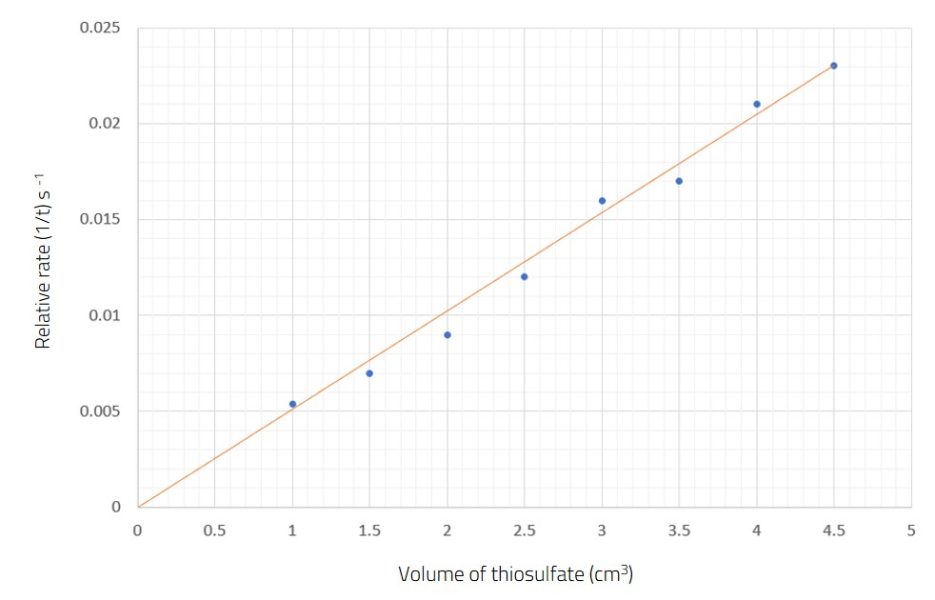
The results in figure 2 demonstrate that the relationship between the volume of thiosulfate solution and the relative rate of reaction is directly proportional. Since the total volume is always the same, the volume of thiosulfate solution can be taken as a measure of thiosulfate-ion concentration. An extension activity for older students could involve calculating the concentrations of thiosulfate ions and plotting concentration against relative reaction rate.
Collison theory can be discussed with students. Increasing the concentration results in more collisions between particles, since there are more particles occupying the same volume of space.
The data points for the slowest reactions have a higher chance of error and anomalous results because the time to obscure the cross is more gradual. This is a good discussion point and allows students the opportunity to repeat reactions and use averages, if desired.
This experiment can be done with larger volumes or with fewer reactions, depending on time and resources. An Arrhenius plot of the data collected can also be drawn, and Arrhenius equations can be used to determine the activation energy for the reaction, which is approximately 50 kJ/mol.[3] If microwell plates are available, the reaction can be done using dropper bottles instead of syringes.[4]
This activity can be extended to investigate the effect of temperature on the relative rate of reaction. By immersing the vial in hot or cold water, the reaction mixture can be heated or cooled quickly. The temperature of the reaction should be below 60°C to minimize exposure to sulfur dioxide.[5]
The aim of this experiment is to find the effect of varying temperature on the relative rate of reaction between ethanedioic (oxalic) acid and an acidified solution of potassium permanganate:
Initially, the reaction mixture is purple in colour due to the presence of permanganate ions, but it will turn colourless as soon as they are used up. This colour change allows us to follow the course of the reaction. The use of dropper bottles allows the use of smaller volumes without sophisticated measuring apparatus and gives students more time to focus on the content rather than measuring accurately.
Some of the chemicals are harmful; wear eye protection. If any chemical splashes on skin, it should be washed off immediately with water.

A sample table of results is shown below and the corresponding graph can be drawn (figure 4).
| Temperature of reaction mixture at start of reaction (°C) | Time for reaction to go colourless (s) | Relative rate, 1/t (s−1) |
| 34 | 78 | 0.0128 |
| 40 | 51 | 0.02 |
| 50 | 25 | 0.04 |
| 60 | 11 | 0.09 |
| 70 | 5 | 0.2 |
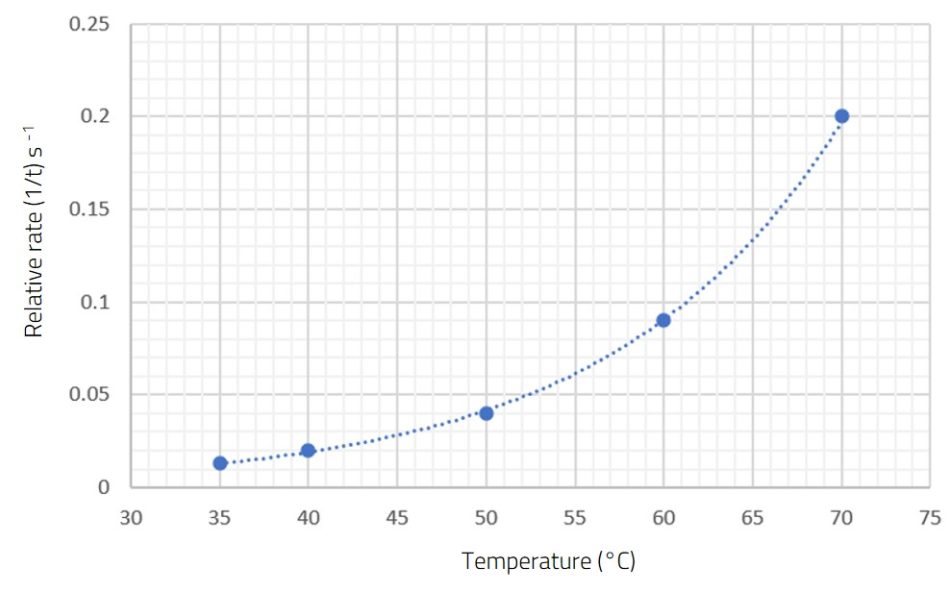
The results in figure 4 demonstrate that the relative rate of reaction increases with rising temperature. However, since the graph of rate against temperature is a curve, the rate is not directly proportional to temperature. In fact, it can be seen from the graph that the rate of reaction doubles if there is a temperature rise of about 10°C.
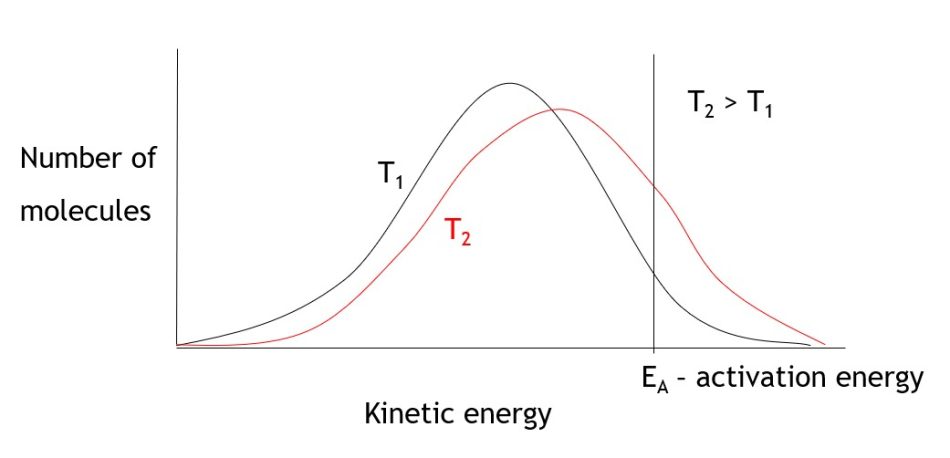
These results can be used to discuss with students the concept of activation energy, which is the minimum energy required for particles to have a successful collision. Diagrams showing the energy distribution of particles can be used to show how a small increase in temperature can give a significant increase in reaction rate, as more molecules will have an energy greater than the activation energy (figure 5).
We would like to thank and acknowledge Howard Tolliday at Dornoch Academy, UK, for his advice and assistance in developing the equipment and his help in collecting the images and video accompanying this article.
[1] Worley B (2021) Little wonder: microscale chemistry in the classroom. Science in School 53.
[2] The Royal Society of Chemistry resource to teach reaction dynamics and mechanisms: https://edu.rsc.org/cpd/teaching-rates-of-reaction-post-16/4013857.article
[3] Worley B, Paterson D (2021) Understanding Chemistry through Microscale Practical Work pp 58–59. Association for Science Education. ISBN: 978-0863574788
[4] The Science on Stage webinar on microscale chemistry: https://youtu.be/LM97yXJlotQ?si=e_IGnqLuTiJdPV84
[5] A video of a small-scale thiosulfate acid reaction with explanations: https://science.cleapss.org.uk/resource/thiosulfate-acid-reaction-rate-and-temperature.vid

Good things come in small packages: discover how microscale experiments can have a big impact in STEM…

Tea is a refreshing drink – and it can also help students to learn about important chemical reactions, as these simple experiments with infusions…

Integrate key principles from biology, chemistry, and engineering with a set of experiments based on…