Supporting materials
Download
Download this article as a PDF

This activity was presented at the Science on Stage Festival 2022. ![]()
Spinning a yarn: explore the chemistry of wool and use it as a raw material for biobased products through simple hand-on activities.

Wool has always been a product of animal husbandry, especially of sheep and goats, and has been used for various purposes, including clothing, mattress stuffing, and thermal insulation.
Since 2002, as a result of European legislation, Regulation EC 1774/2002, later revised in 2009 (EC1069/2009), wool went from being classified as an agricultural product to being classified as processing waste, so it has to be disposed of through very expensive procedures.[1]
According to statistics reported by the International Wool Textile Organisation (IWTO),[2] the number of sheep farms is increasing as a result of interest in meat, and wool production is also on the rise, since, for their welfare, the animals must be sheared.
To avoid having to send wool for disposal, industries and research centres have been developing several strands of study over the years, investigating alternative uses for wool. Material derived from wool can be used as adsorbent or insulating elements, and as starting materials for filters or other biomaterials.[3,4]
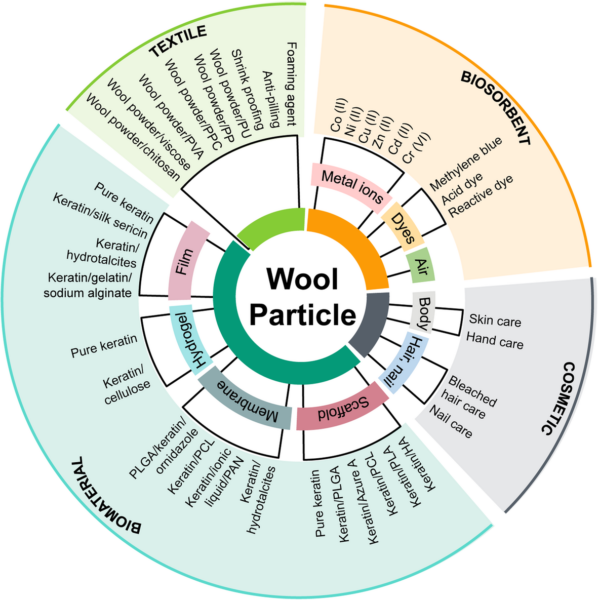
Wool is a natural fibre that consists of proteins (about 95–98%, of which about 85% is keratin), lipids (about 2%), and mineral salts (1%). Keratin is a very abundant protein in nature that is found in hair, nails, horns, and bird feathers, in addition to wool.[5,6]
It is reported in some works that the first use of keratin for medical purposes dates back to China in 1650, but the term was first reported in the scientific literature in 1850 to explain the composition of the horns of some animals.[7]
The activities can be incorporated into different curricular tracks:
The in-depth interdisciplinary laboratory activities can be offered to students aged 14–16 and 16–19.
The goals are to engage students in active learning and stimulate their critical thinking.
This activity involves the extraction of keratin from wool using a suitable extraction solution.
It can be completed in one or two lessons; the extraction can be carried out for an hour with 1 M NaOH or overnight with 0.1 M NaOH.
Keratin is a fibrous protein that, in terms of secondary structure, can be organized as an alpha helix or beta sheet. The high content of cysteine residues promotes the formation of disulfide bridges that add to hydrogen bonds, and ionic and hydrophobic interactions contribute to the three-dimensional (3D) organization of the protein. Depending on the origin of keratin, the 3D structure and specific amino acid content changes, but the main inter- and intramolecular bonds remain the cystine disulfide bridges.
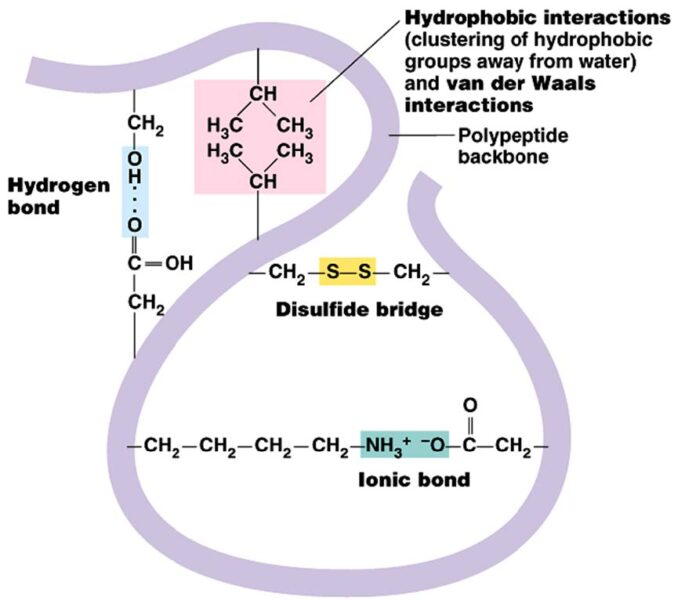
Keratin extraction depends precisely on breaking of the abovementioned interactions. Depending on the extracting agent, different amino acid compositions of the extracted keratin and different chemical and physical characteristics will be obtained. For this reason, considering the possibility of employing keratin in the production of new biomaterials, different fields of research have been developed. In recent years, with the aim of developing increasingly effective extraction techniques and production methods for hydrolyzed keratin, which vary according to the characteristics of the product required and its use, several new keratin-based products have been developed: films, adsorbent sponges, polymeric fibres, particular applications in the field of biomaterials.[7,9–12]
Dilute 0.1 M solutions of sodium hydroxide are not classified as hazardous, but 1 M sodium hydroxide is corrosive. Gloves and eye protection should be worn to avoid eye and skin contact. Working under a fume hood is ideal.
Teachers should make up the solutions so that students don’t use the more concentrated forms.

During the experiments, students should record their observations on the worksheet and afterwards the answers should be discussed with the class.
The wool generally takes on a darker and gelatinous appearance during extraction and the clear solution becomes dark and cloudy. These changes are due to chemical reactions.
As described above, the 3D shape of proteins depends on the numerous bonds formed between different peptide chains and within the same protein chain. These are generally weak bonds (hydrogen bonds, hydrophobic interactions, dipolar bonds), which are strongly influenced by external factors, such as pH, temperature, the presence of ionic salts, emulsifiers, the activity of certain enzymes, and the action of certain microorganisms.
Alkaline hydrolysis of wool produces 75−80% water-soluble materials, including amino acids and peptides.[11] Upon treatment with alkali solutions, different chemical reactions occur to denature the proteins and disrupt the 3D structure through breakdown of the hydrogen and disulfide bonds, ionization of the carboxylic groups of amino acids, and changes in solubility. The use of alkali solutions can also lead to the formation of a sulfide odour during the process.[9]
In this activity, the effects of different chemicals on the flocculation of keratin are investigated. The activity takes about 30 minutes to complete.
Once the solution containing the extracted protein is obtained, it can be precipitated by using several methods: addition of salts to change the ionic strength or changing the solvent polarity, temperature, or pH.
The moment the protein is denatured, it ‘opens up’ and interactions that were previously intramolecular become intermolecular, aggregating the protein and increasing its hydrophobic character. This decreased affinity to water can evolve toward aggregation and polymerization phenomena, which can be
– disordered, giving rise to precipitation, flocculation, and coagulation
– ordered, generating more or less stable gels.
Precipitation is characterized by the loss of solubility alone, due to association phenomena and consequent separation of proteins from solution.
Flocculation occurs when floccules, that is, large micellar aggregates, are formed, without denaturation of the protein structures. This occurs when protein micelles of colloidal size no longer exhibit electrostatic repulsions, and thus, aggregate.

Flocculation of proteins can be achieved 1) by changing the pH by adding weak acid solutions, and 2) through treatment with organic solvents miscible with the aqueous phase (such as ethanol or acetone) in which the proteins are dissolved.
Dilute 0.1 M solutions of sodium hydroxide are not classified as hazardous, but 1 M sodium hydroxide is corrosive. Gloves and eye protection should be worn to avoid eye and skin contact with the solutions. Working under a fume hood is ideal.
Teachers should make up the solutions so that students don’t use the more concentrated forms.

Ask students what differences they notice when flocculation is induced by pH or solvent changes. Can they think of explanations for why pH or solvent changes might cause flocculation?
Hydrolysed keratin produced by wool is mainly used in cosmetics as an ingredient in hair-specific products.
The final proposed activity is to make hair conditioner using keratin. For better success in the classroom, it is best to buy keratin as a 25% (m/V) solution glycerine. It is difficult to use the extracted keratin because it is not sufficiently soluble in water and needs to be purified.
This activity takes 30 minutes to complete.
The solutions used are not particularly hazardous, but ideally gloves and eye protection should be worn.
For 50 ml of product:

Students should understand that it is possible to use materials produced from waste to produce cosmetics, which is better more sustainable using virgin materials. As an extension activity, the different aspects of the circular economy of wool could be researched in more detail.
The proposed activities provide serval useful aspects for teaching:
These are simple experiments that only barely touch the extensive literature related to the use of biomass from waste material, but they start from a material that is known to all and easy to find. The reagents and materials are also not difficult to find, even in a poorly equipped chemistry laboratory.
Other interesting keratin activities can be found on the Science on Stage website.
The project presented in this paper grew out of a collaboration with researcher Annalisa Aluigi and CNR Biella. Subsequently, some activities were developed with Science on Stage coordination and are part of the unit ‘The 3 Rs and the Products of the Future’ created within the project https://www.science-on-stage.eu/act-now-sdg
[1] Petek B, Logar RM (2020) Management of waste sheep wool as valuable organic substrate in European Union countries. Journal of Material Cycles and Waste Management 23: 44–54. doi: 10.1007/s10163-020-01121-3
[2] A summary of the number of sheep and wool production in the world: https://iwto.org/wp-content/uploads/2022/04/IWTO-Market-Information-Sample-Edition-17.pdf
[3] Sun Y et al. (2022) The progress and prospect for sustainable development of waste wool resources. Textile Research Journal 98: 468–495. doi: 10.1177/00405175221098572
[4] Zhang C et al. (2020) Utilization of waste wool fibers for fabrication of wool powders and keratin: a review. Journal of Leather Science and Engineering 2. doi: 10.1186/s42825-020-00030-3
[5] Vineis C et al. (2019) Extraction and characterization of keratin from different biomasses: extraction from waste biomass and applications. In Sharma S, Kumar A (eds) Keratin as a Protein Biopolymer pp 35–76. Springer. ISBN: 978-3-030-02900-5
[6] Stephen G et al (2022) Wool keratin as a novel alternative protein: a comprehensive review of extraction, purification, nutrition, safety, and food applications. Comprehensive Reviews in Food Science and Food Safety 22: 643–687 doi: 10.1111/1541-4337.13087
[7] Koleva M, Zheleva D (2022) Methods for obtaining of keratin hydrolysates from sheep wool. Journal of Chemical Technology and Metallurgy 57: 76–83.
[8] Gaidau C et al. (2021) Wool keratin hydrolysates for bioactive additives preparation. Materials 14: 4696. doi: 10.3390/ma14164696
[9] Shavandi A (2017) Keratin: dissolution, extraction and biomedical application. Biomaterials Science 5: 1699–1735. doi: 10.1039/C7BM00411G
[10] Burnett CL et al. (2021) Safety assessment of keratin and keratin-derived ingredients as used in cosmetics. International Journal of Toxicology 40: 36S–51S. doi: 10.1177/10915818211013019
[11] Cardamone JM et al. (2009) Characterizing wool keratin. Advances in Materials Science and Engineering 147175. doi: 10.1155/2009/147175
[12] Banasaz S, Ferraro V (2024) Keratin from animal by-products: structure, characterization, extraction and application—a review. Polymers 16: 1999. doi: 10.3390/polym16141999
Wool is a fascinating biomaterial and has been used from the early history of mankind until our days. The author gives teachers and especially their pupils the opportunity to examine the chemistry of wool based on interesting and activating experiments. The novelty here is the woolly combination of chemistry and circular economy.
Ernst Hollweck, biology & chemistry teacher. Staatliches Gymnasium Holzkirchen, Germany.
Download this article as a PDF

Act now for the Sustainable Development Goals: explore resources developed by European teachers bring the science of sustainability into the classroom.
How can AI systems like those developed to beat humans at games help unlock the secrets of protein…

When life gives you lemons: use limonene to explore molecular properties with your students and show them the scientific method in…