Supporting materials
Download
Download this article as a PDF

This activity was presented at the Science on Stage Festival 2022 ![]()
When life gives you lemons: use limonene to explore molecular properties with your students and show them the scientific method in action.

The activities in this article allow students to investigate simple molecular properties using an easily accessible compound: limonene. The activities were developed by groups of Italian and Spanish students in a joint project through written assignments, questions, and videos.
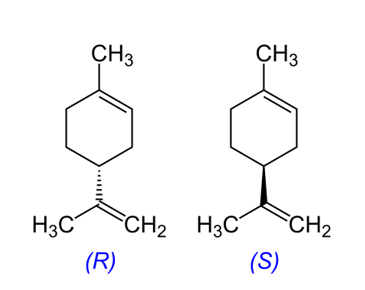
Through the application of the scientific method, students develop the ability to observe a phenomenon, formulate a hypothesis, search for suitable materials to set up an experiment, collect data for analysis, and arrive at a conclusion that is shared with others. The focus here is on a simple organic molecule, (R)-(+)-4- and (S)-(–)-4-isopropenyl-1-methyl-1-cyclohexene (the two enantiomers of limonene), which is safe, easy to find, and of real-world and industrial interest.
Limonene (4-isopropenyl-1-methylcyclohexene) is a chiral cyclic monoterpene, with the molecular formula C10H16.[1–3] This chiral compound exists in two isomeric forms, which are stereoisomers; in other words, molecules with the same structure but with different orientations of the same atoms in space. The two forms of limonene differ only in the configuration of the groups around a single carbon atom. The two forms are mirror images (=enantiomers) of each other that can only be interconverted by breaking and forming covalent bonds.
Molecular Formula: C10H16
Molecular Weight: 136.23
Synonyms:
D-Limonene, (R)-(+)-Limonene
L-Limonene, (S)- (−)-Limonene
D-Limonene is a major component of the oil in citrus peels, which also contains citrus limonoids.[4] The less common L-limonene is found in herbs like caraway. Industrial limonene comes from the peel of citrus fruits as a waste product of fruit processing for the production of juices.[5] For this reason, it is produced as the R enantiomer (D-limonene). It is used as a fragrance compound and for degreasing.

Like all enantiomers, the two forms are indistinguishable in most chemical/physical tests. They do, however, rotate the plane of polarized light in opposite directions. Thus, it is possible to distinguish the enantiomers by using a polarimeter.
Furthermore, they are easily distinguished biologically. D-limonene (the R form) has a sweet citrus odour, while the L form has a sharper resinous or turpentine-like odour. Our noses are very sensitive and able to distinguish these very similar compounds because the olfactory receptors in the nose have molecular sites that interact specifically with different enantiomers.[6]
Experiments:
Extension activities: the antibacterial and anti-germination properties of limonene (2 h)
Limonene is a volatile terpene, which, if concentrated, must not be swallowed or come into contact with the eyes because it is an irritant. It must be immediately washed from the skin. Unlike in orange peel, the limonene content in resin and turpentine does not reach critical concentrations.
In the first activity, we discuss chirality using (R)- and (S)-limonene. This activity should take around 60 min.
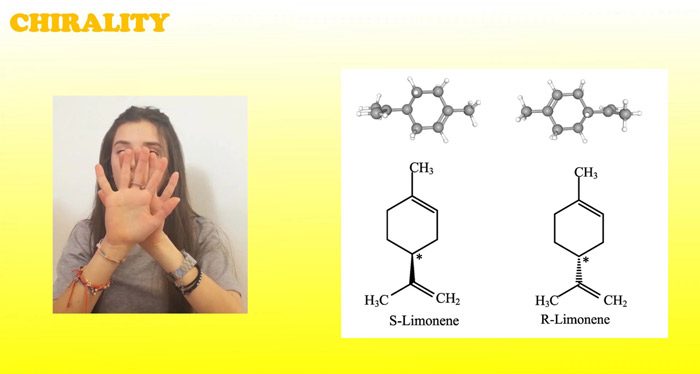
Next, students can investigate chirality using (R)- and (S)-limonene and see how this very minor structural change can have real-world consequences. It should take around 60 min.
The specific rotation angle to recognize each enantiomer is +/− 123°, but, in reality, it is sufficient to observe that the R enantiomer is extinguished by rotating the analyzer to the right, while, for the S enantiomer, rotation is in the opposite direction with the same angle.
The smell of citrus also depends on the citrus limonoid molecules. We have added references and a theoretical activity to understand the difference between these molecules and study the metabolic pathways in plants.
The perception of the smell of (R)-limonene as being different from that of (S)-limonene is common but not universal. Students could design an experiment to test this, for example, to determine the percentage of the population in a sample group able to distinguish them.
Next, students can investigate the effect of limonene on different polymers. This activity should take around 1–2 h. All steps should be performed by the students. There are quite a lot of tests, so, if necessary, students can work in groups to save time/equipment.
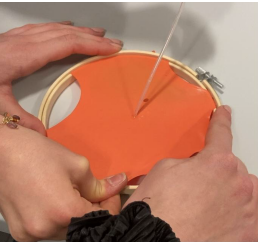
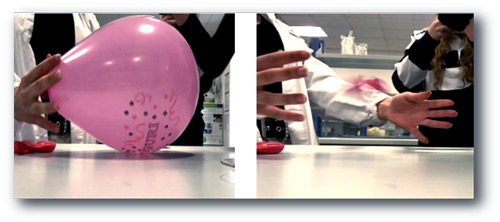
Latex material, made up of 30–40% cross-linked polyisoprene, reacts with limonene, ‘opening’ a hole around the drop or touch point. This is because the limonene molecules (monoterpenes) are small enough to slip along the polyisoprene chains and move them apart, loosening the chemical curing. This is enough to snag the sample and pop the gas-filled balloon. The reaction is proportional to the concentration of limonene and to the thickness of the rubber, but it does not differentiate between (R)- and (S)-limonene.
In this activity, we investigate the chemical properties of limonene. It should take around 30 min.

Limonene is soluble in oil and mixes in it naturally: it is deduced that it is a nonpolar solute that dissolves in a nonpolar solvent.
Limonene is insoluble in water and does not mix with it: limonene is a nonpolar molecule that does not dissolve in water, a polar molecule.
The polarity of a molecule depends on the chemical species that form it, on the difference in electronegativity present in the covalent bonds, and on the fact that the centre of the positive electrostatic forces does not coincide with the negative ones.
Limonene is a hydrocarbon consisting of carbon and hydrogen atoms. It is a monoterpene, an isoprene derivative, with a closed chain in which the only areas with a weak partial negative charge are double bonds; these charges are too weak to result in an interaction with water molecules.
[1] Erasto P, Viljoen AM (2008) Limonene – a review: biosynthetic, ecological and pharmacological relevance. Natural Product Communications 3: 1193–1202. doi: 10.1177/1934578X0800300728
[2] Perveen S (2018) Introductory Chapter. In Perveen S, Al-Taweel A (eds) Terpenes and Terpenoids pp 1–12. IntechOpen. ISBN: 978-1-78984-777-2
[3] Chemical information for limonene and a virtual 3D model: https://pubchem.ncbi.nlm.nih.gov/compound/Limonene#section=3D-Conformer
[4] Gualdani R et al. (2016) The chemistry and pharmacology of citrus limonoids. Molecules 21: 1530. doi: 10.3390/molecules21111530
[5] Jongedijk E et al. (2016) Biotechnological production of limonene in microorganisms. Applied Microbiology and Biotechnology 100: 2927–2938. doi: 10.1007/s00253-016-7337-7
[6] Malnic B, Godfrey PA, Buck LB (2004) The human olfactory receptor gene family. PNAS 101: 2584–2589. doi: 10.1073/pnas.0307882100. This article has a correction.
Download this article as a PDF