Elements in focus: beryllium Understand article
As a lightweight, super-strong metal, beryllium is an engineer’s dream – but it also has some less convenient qualities.
fourth element in the
periodic table
Antoine2K/Shutterstock.com
The element beryllium is perhaps one of the strangest in the periodic table. It is the second lightest metal (by atomic mass), after lithium, and it is also surprisingly unreactive compared to its elemental neighbours. Beryllium is the first element in Group 2, and – unlike the other alkaline earth metals (such as magnesium, calcium and strontium) – it resists interacting with most other substances. In fact, this dark-grey metal would be one of the most useful elements in the periodic table if it were more abundant and not so dangerous to work with.
Alloys and applications
Beryllium’s chemical stability is due to its tendency to form a very thin, unreactive oxide layer on its surface. This and its low density are advantages in making very strong, corrosion-proof metal alloys, such as beryllium copper. These alloys have uses in high-stress applications, such as helicopter rotors. They are also used as an alternative to steel in some tools – especially where there are high magnetic fields, such as from radio transmitters or magnetic resonance imaging (MRI) equipment in hospitals.
Pure beryllium is also used in some specialist applications, despite its high cost, because of its superb engineering qualities. As well as its lightness and strength, beryllium has a high melting point and relatively low thermal expansion, so it changes very little when heated – or when cooled to exceptionally low temperatures, such as those in space. These qualities have made beryllium the material of choice in many aerospace applications, including for parts of satellites and supersonic aircraft. The James Webb Space Telescope (JWST) – a new space telescope to replace the Hubble – will have mirrors made from pure beryllium. The JWST is expected to be launched in 2021, and once in place, the 18 hexagons of beryllium that make up the telescope’s primary mirror will be kept cooled to –220°C. This is to minimise infrared emissions from the mirror itself that could otherwise interfere with signal detection.

Ball Aerospace/Flickr, CC BY-NC-ND 2.0
Beryllium has another special property: unlike most metals, X-rays can shine straight through it. This transparency to X-rays is due to the low number of electrons in its atoms: as element number 4 in the periodic table, it has just four electrons in each of its atoms (along with four protons and usually five neutrons within the nucleus). Electrons interact with passing X-rays, deflecting or absorbing them and releasing their own secondary X-rays. Since beryllium has only four electrons, X-rays can whizz past its atoms with little chance of coming into contact with the electrons. Although the element lithium – with atomic number 3, and thus even fewer electrons – would in theory make an even better X-ray ‘window’, it is far too reactive to use in any structural applications, unlike beryllium.
Beryllium facts
- Element name: Beryllium
- Symbol: Be
- Atomic number: 4
- Relative atomic mass: 9.01
- Density: 1.85 g/cm3
- Melting point: 1287°C
- Periodic table group: 2 (alkaline earth metals)
- Abundance in Earth’s crust: 2–6 parts per million
- Discovery: Minerals containing beryllium (such as beryl and emerald) have been known since ancient times. The element itself was identified in 1798, but the name beryllium was not used until 30 years later.
Letting the X-rays shine through
Beryllium’s unusual property of transparency to X-rays means that it can be used to enclose samples for analysis using X-rays, as the rays can pass through the enclosure without much disturbance. This is exceptionally useful in research facilities that use X-rays to study matter at the atomic level, such as the European Synchrotron Radiation Facility (ESRF)w1 and the European X-Ray Free-Electron Laser (European XFEL)w2. When intense pulses of X-rays are fired at the samples under investigation, the way the X-rays are scattered or absorbed provides data about the properties and behaviour of the atoms in the sample, which scientists can then interpret. At European XFEL, a finely focused X-ray beam is used to target samples such as individual viruses and biomolecules. At ESRF, the beam enables scientists to study the properties and molecular structure of matter in extremely high detail, leading to the development of new materials, or new uses for existing ones.
In intense X-ray sources like those at ESRF and European XFEL, some parts of the apparatus need to be isolated within airtight vacuum conditions. But putting up barriers to air flow would obstruct the path of the X-rays – unless the barriers are made of beryllium. Beryllium also shields the X-ray detectors from other particles that might interfere with readings – for example, in the spectrometer used to monitor the properties of the X-ray pulses at European XFEL.
Focusing with beryllium lenses
Just as transparent glass is used to focus visible light, beryllium is used to make lenses that focus X-ray beams. When a beam is focused to a fine point, there are more X-ray photons in a smaller space, which increases the effect of the X-rays interacting with the experimental samples.
To focus X-rays, beryllium lenses need to be concave – curving in the opposite direction to the familiar convex lenses used to focus visible light. Because the refracting power of beryllium is very low, the lenses are generally used as stacks of individual lens elements, which are known as compound refractive lenses (CRLs). These stacked lenses produce an impressive focusing power: the final width of the X-ray laser beam can be as little as a fraction of a micron (10-6 m) across – some 10 000 times sharper than the initial diameter of the beam.
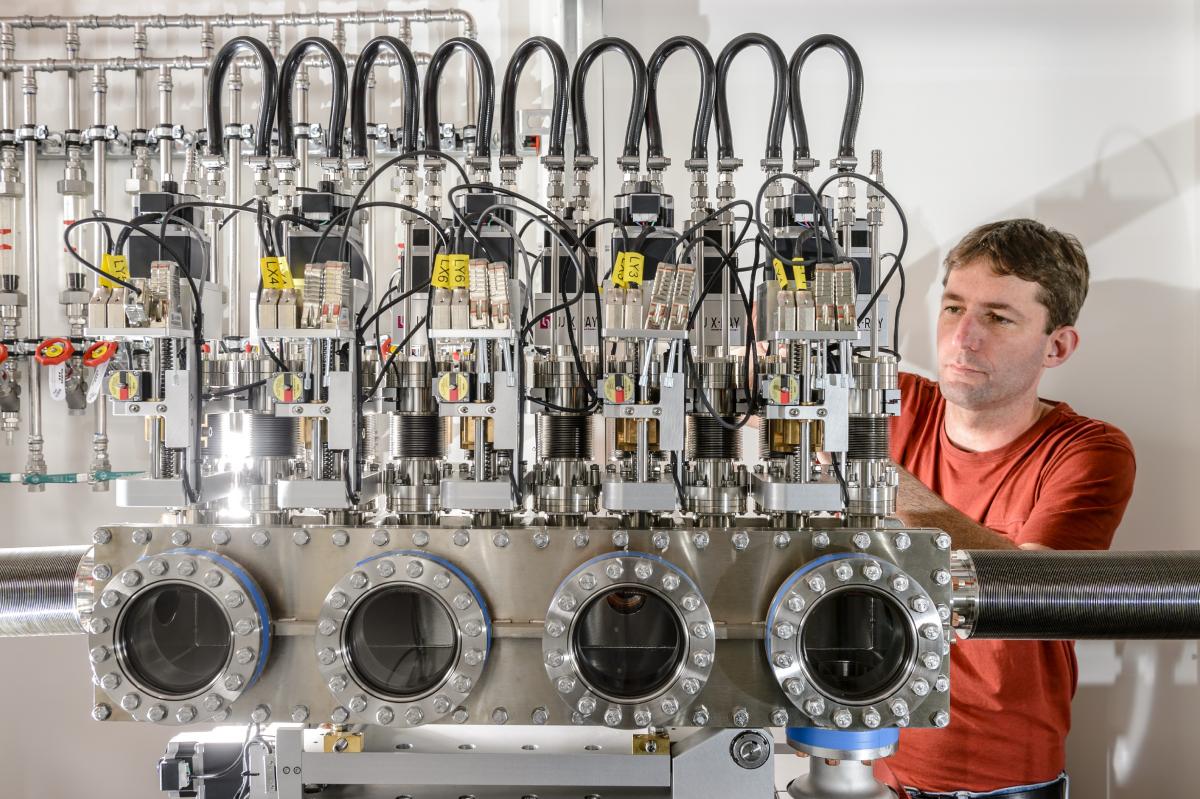
European XFEL
Rare and dangerous
But as well as these exceptional qualities, beryllium has a troublesome side. First, the element is scarce: it is found only in rare minerals, such as beryl – the type of gemstone that includes emeralds and aquamarines. This scarcity makes beryllium expensive, so it is used only when absolutely necessary. Yet where it is used, it’s there to stay: beryllium components within X-ray research facilities could last hundreds of years before they need to be replaced.

greyloch/Flickr, CC BY-SA 2.0
Another problem is that beryllium is notoriously toxic. Breathing in just a small amount of beryllium dust can cause berylliosis – a disease with persistent, incurable symptoms similar to pneumonia, and which may lead to cancer. As a result, in situations where beryllium metal is used, special precautions need to be taken to keep dust from entering the general environment. Shaping the metal is thus difficult, because the dust produced when machining beryllium is so toxic. For this reason, beryllium lenses are made by pouring molten beryllium into standardised moulds, rather than by grinding the metal into shape.
So while beryllium is tantalizingly versatile, its rarity and the risks it poses mean that it can be considered only for the most select and costly applications. And there is always a need to respect the inherent dangers of this highly unusual metallic element.
Discovery of the neutron
Beryllium played a crucial role in the discovery of the neutron in 1932. A few years earlier, several scientists had observed that beryllium produced emissions when bombarded with alpha particles. Because the emissions had no charge (unlike the only known subatomic particles, electrons and protons), the scientists concluded that they must be gamma rays – high-energy photons. However, the energy of the emissions was not what would be expected for gamma rays. In 1932, British scientist James Chadwick repeated the experiment and concluded that the emissions must be a new subatomic particle with no charge – the neutron. From this work he was also able to calculate the mass of the new particle, which he found was similar to that of the proton – just as we know today. In 1935, Chadwick was awarded the Nobel Prize in Physics for his discovery.
Acknowledgement
The authors would like to thank Dr Peter Zalden, instrument scientist at European XFEL, for his assistance in providing information for this article.
Web References
- w1 – Situated in Grenoble, France, ESRF is the world’s most intense X-ray source and a centre of excellence for fundamental research in condensed and living-matter science. The Extremely Brilliant Source project, planned for completion in 2022, will be a new source 100 times greater in brilliance and coherence for better understanding of materials.
- w2 – European XFEL is a research facility in the Hamburg area in Germany. Its extremely intense X-ray flashes are used by researchers from all over the world to study the structure and behaviour of materials at the atomic level and at ultrafast time scales.
Resources
- For more information about beryllium and its uses, check the website of the Beryllium Science and Technology Association (BeST).
- Read a short book all about beryllium and its applications. See:
- Adair R (2007) Beryllium. Rosen Central, New York. ISBN: 1404210032
- Watch a video on beryllium and its uses, which includes an explanation of a beryllium ‘window’ at the MAX-lab synchrotron in Sweden, as part of the University of Nottingham’s ‘Periodic Table of Videos’ series.
- Find out more about the James Webb Space Telescope, a collaboration between NASA, the European Space Agency (ESA) and the Canadian Space Agency. Learn more about the primary mirror made from beryllium on the NASA website.
- Watch a video from the UK’s Royal Institution about beryllium and the JWST.
- Read about the discovery of the neutron on the American Physical Society website.
- For information on all the chemical elements, read this book:
- Gray T (2009) The Elements: A Visual Exploration of Every Known Atom in the Universe. Black Dog & Leventhal Publishers, Inc., New York. ISBN: 1603764054
Institutions
Review
In the periodic table, there are some little-known elements that have a lot of applications. This article could deepen students’ knowledge about one such metallic element: beryllium.
Before reading the article, students could think about the metals they know and how their applications have changed throughout history. The article could then be used as a starting point for investigating and discussing the uses of different rare elements in our society. It could also help to raise awareness of the huge cost of obtaining them and the importance of recycling.
Potential comprehension questions include:
- What is beryllium’s atomic number and what is its position in the periodic table?
- Why is beryllium so different from the other elements of its group?
- Can you list five of beryllium’s properties?
- Can you describe in detail three of beryllium’s applications?
- Why is beryllium used only when absolutely necessary?
- Can you explain what role beryllium played in the discovery of the neutron?
Mireia Güell Serra, chemistry teacher, INS Cassà de la Selva school, Spain





