Turning dandelions into rubber: the road to a sustainable future Teach article
A species of dandelion is leading the way towards sustainable rubber. Find out how, by growing this unusual plant yourself and extracting the rubber from the roots.
Look around you: what objects can you see that are made from rubber? You might spot an elastic band, a pencil eraser, or perhaps one of the biggest users of rubber – car tyres. In fact, over 40 000 everyday products are produced from natural and synthetic rubber. With this material in such high demand, scientists are exploring an alternative source of rubber from a particular species of the familiar, but underestimated, dandelion: Taraxacum kok-saghyz, commonly known as the Russian dandelion.

Mareike Göbel
To help students understand more about sustainability and this unusual source of rubber, we developed two simple methods to extract the rubber from the dried roots of the Russian dandelion. In the first method, students grind the roots into a powder to extract the rubber particles and create their own eraser. In the second method, sodium hydroxide is used to erode the woody parts of the root, and students can stretch out the fine strands of rubber that remain. The activities can be carried out by students aged 11–14 or older, in small groups of three or four, and will take about two hours.
Rubber: sources and structure
The majority of the world’s natural rubber comes from plantations of the tropical rubber tree Hevea brasiliensis – over 90% of which are cultivated in Southeast Asia. Natural rubber production is responsible for widespread deforestation, and most of this rubber is used to make tyres. Relying on natural rubber to meet rising demands is not only unsustainable, but also risky: yields of natural rubber are falling due to a decline in prices, which has resulted in farmers switching to more profitable plantations such as palm oil. Climate change and crop diseases pose additional threats. These challenges make the future of natural rubber production uncertain. Producing rubber synthetically can help, but it requires the use of crude oil – so it is important to find an environmentally friendly and sustainable alternative.

Mareike Göbel
The discovery of Russian dandelion
The Russian dandelion, Taraxacum kok-saghyz, was discovered in Eastern Kazakhstan in 1931. The plant has the yellow flowers characteristic of the dandelion genus, but the roots contain a higher percentage of rubber than the familiar species of dandelion found in Europe. Soon after its discovery, the Soviet Union began large-scale cultivation of T. kok-saghyz to extract the rubber and end the country’s dependence on imports (see Göbel & Gröger, 2016). When the Japanese seized rubber plantations in Southeast Asia during World War II, other countries, including the USA and Germany, started farming the dandelion as an alternative. When the war ended and supplies from the rubber tree, H. brasiliensis, became available again, research on the Russian dandelion ceased – until recently.
A replacement for natural rubber
Today, research into the Russian dandelion is growing. The Fraunhofer Institute for Molecular Biology and Applied Ecologyw1, based at the University of Münster, and the tyre company Continentalw2 are just two of the organisations working on using Russian dandelion as an alternative source of rubber.
Compared to tree-sourced rubber, the Russian dandelion is extremely resilient and can be grown in moderate climates on poor soils. This means that it can be grown locally, reducing dependence on imports. It is also much quicker to grow dandelions than trees – Russian dandelions can be harvested twice a year, whereas natural rubber trees take 7–10 years to mature before the sap, which is processed into rubber, can first be collected. What’s more, the Russian dandelion produces inulin (a sugar substitute and potential biofuel) as a by-product.
Structure and properties of rubber
Rubber is used in so many products because of its unique chemical and physical characteristics. An elastomer (a polymer with elastic properties, such as rubber) can be stretched to over 100% of its original length without breaking. Natural rubber consists of long chains of cis-1,4-polyisoprene (figure 1), each comprising up to 30 000 isoprene monomers. The chains are interwoven with one another like a plate of cooked spaghetti. When you stretch rubber, the chains are pulled apart but do not break, due to the linkages between the entangled chains (figure 2).
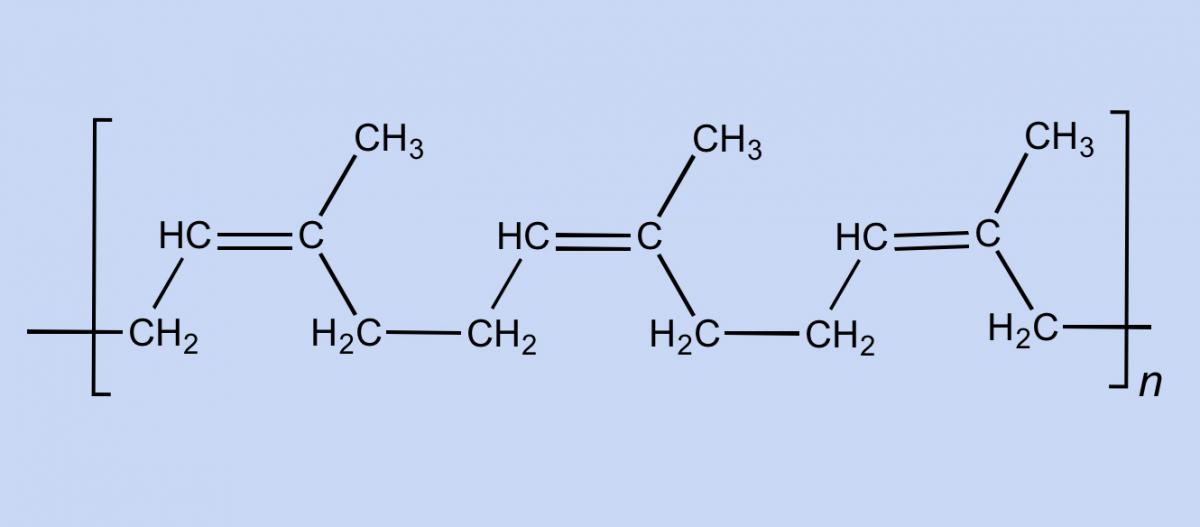
Smokefoot/Wikimedia Commons
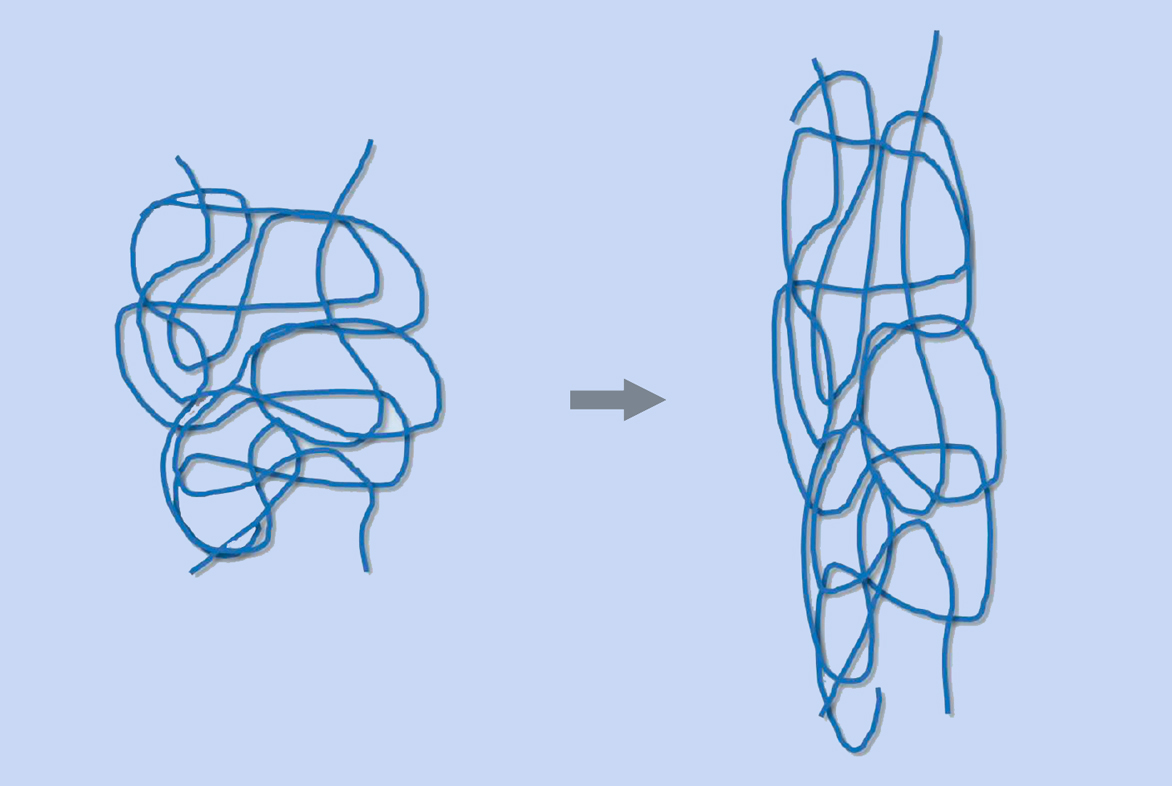
Mareike Göbel
Preparing the plant material
In preparation for the following activities, you will need to grow the Russian dandelion plant and harvest the roots to dry. The whole preparation process will take approximately seven months from February to September, but teachers could use this opportunity to teach students about plants and the requirements for growing them. Seeds can be purchased onlinew3 and should be sown in February in a window-sill greenhouse. After two months, transfer the plants into flower boxes. You can harvest the roots from August onwards. To dry them, place the roots on paper towels and leave them at room temperature for 3–4 weeks.
Introductory activity
Before carrying out the two main activities, spark your students’ interest with this starter activity introducing the topic of rubber and the Russian dandelion.
Procedure
- Ask your students to list rubber products that they use in their everyday lives.
- Show students a photo of the Russian dandelion and ask them to find the connection between the dandelion and the rubber products. To prompt them, ask where natural rubber usually comes from.
- Give each student one dried Russian dandelion root. Instruct them to carefully break the root in half so they can see the very fine elastic rubber threads within the root (figure 3). Note that these threads can rip easily if the root is broken too forcefully.

Mareike Göbel
Activity 1: Making a dandelion root eraser
In this activity, students physically extract rubber from dried Russian dandelion roots to produce a small eraser.
Materials
Each group of 3–4 students will need:
- 3–4 pieces of dried Russian dandelion root. Note: you should choose roots with a diameter of about 1 mm, and break them into pieces of about 2–3 cm in length.
- Pestle and mortar
- Tweezers
- Watch glass
- Water
Procedure
- Place the dried dandelion roots into the mortar (figure 4). The greater the number of roots you have, the more time and effort is required to grind them.
- Using the pestle, grind the roots for 5–10 minutes until they become a homogeneous powder (figure 5).
- As you grind the roots, remove any small, globular brown particles in the mortar using tweezers, and place aside on the watch glass. These are the rubber particles.
- Continue grinding the roots until no further brown particles are formed in the powder.
- Wash out the mortar with some water to remove the woody powder. Place the rubber particles back into the mortar, which should still be slightly wet (figure 6).
- Grind the rubber particles until they stick together (figure 7) and form a piece of rubber about 0.5–1 cm in length (figure 8).
- You can now test the piece of rubber as an eraser. Students can merge the rubber from multiple groups to create a bigger eraser.

Mareike Göbel

Mareike Göbel

Mareike Göbel

Mareike Göbel

Mareike Göbel
Activity 2: Refining the rubber threads
The rubber from fresh dandelion roots is an emulsion of polymer micro-particles, similar to the sap from a rubber tree. In the dried roots, however, the rubber is a solid aggregate. In this activity, students dissolve the outer, woody material of the dried roots to gain access to the inner net of rubber.
Materials
Each group of 3–4 students will need:
- 1–2 pieces of dried Russian dandelion root. Note: you should choose roots with a diameter of about 0.5 cm, and break them into pieces of about 3–4 cm in length.
- 5 ml of sodium hydroxide (NaOH) solution (3%)
- Test tube
- Water bath set to approximately 90 °C
- Tweezers
Safety note
This procedure involves the use of sodium hydroxide solution. Students should wear lab coats and safety goggles. See also the general safety note.
Procedure
- Place a piece of dandelion root in a test tube.
- Cover the root with the sodium hydroxide solution.
- Place the test tube in the water bath for about one hour. The colour of the sodium hydroxide solution will change from clear to brown, but the solution will remain transparent.
- After one hour, remove the root from the test tube using tweezers. The root will now be soft.
- Using tweezers, carefully wash the root by dipping it into the water from the water bath. The woody material of the root will wash away, but if needed, you should remove any remaining tissue using the tweezers until you are left with only the bright white net of fine rubber threads.
- Gently extend the threads by pulling them apart (figure 9).
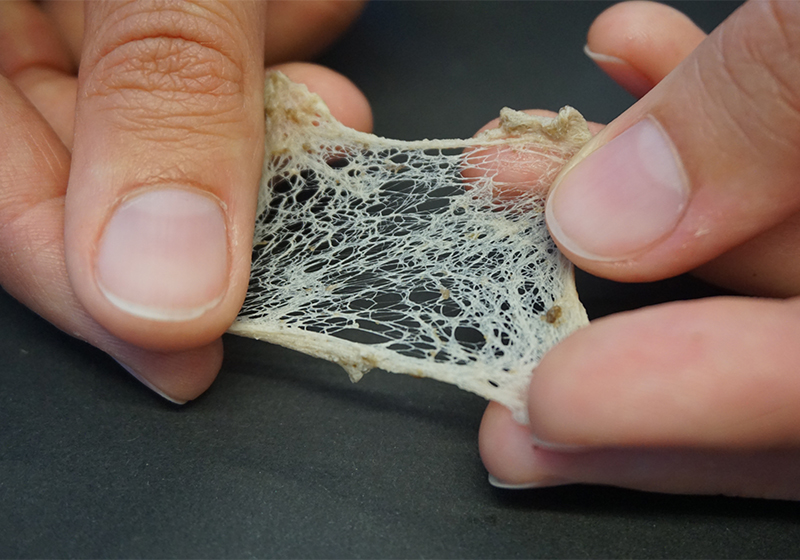
Mareike Göbel
Discussion questions
Alongside the activities, discuss the following questions with your students to teach them about different types of rubber, the properties of rubber, and the importance of sustainability.
- What are the advantages and disadvantages of rubber from Hevea brasiliensis and synthetic rubber?
- Why is it important to look for alternative sources of rubber like the Russian dandelion?
- When you break a dandelion root in half, what can you see?
- What form does the rubber take in dried dandelion roots?
- What are the advantages and disadvantages of dandelion rubber?
- What are the properties of rubber? Explain its elasticity.
Extension activity
Seeing and experiencing the elasticity of rubber from the Russian dandelion is sufficient proof of natural rubber. To extend the activities, however, you could chemically detect the double bonds in polyisoprene, for example using bromine, potassium permanganate (Baeyer test) or the Burchfield colour reaction (see Göbel & Gröger, 2017).
References
- Göbel M, Gröger M (2016) Kautschukforschung am russischen Löwenzahn in der Zeit um den Zweiten Weltkrieg. Praxis der Naturwissenschaften – Chemie in der Schule 8(65): 19–22
- Göbel M, Gröger M (2017) Alternative Kautschukquellen am Beispiel des russischen Löwenzahns experimentell erschließen. Praxis der Naturwissenschaften – Chemie in der Schule 2(66): 26–30
Web References
- w1 – Understand how researchers are using Russian dandelion rubber as an environmentally friendly alternative to the natural rubber tree in this video from the Fraunhofer Institute.
- w2 – Learn how rubber from the Russian dandelion can be used to produce tyres in this video from the tyre company Continental.
- w3 – Seeds of Russian dandelion, Taraxacum kok-saghyz, can be purchased from the All Good Things Organic Seeds website.
Resources
- Understand more about rubber and inulin production from the Russian dandelion in an article from Owlcation.
- Read about the European DRIVE4EU project, which is working to make Russian dandelion rubber a serious alternative to natural rubber.
- The gene editing technique CRISPR-Cas9 has been used to increase the rubber content of Russian dandelion plants. Read more in an article from Chemical & Engineering News.
Review
We live in a time when we must reconsider our habits and how we use natural resources. This article, which provides information on how rubber can be made using a species of dandelion, can be used for chemistry, biology and environmental sciences lessons. It is particularly suitable for discussing sustainability issues related to technological development, and for considering global questions regarding biodiversity.
The article also describes a novel activity using the roots of the dandelion. The Taraxacum species must be grown well in advance, but teachers could grow the plants with students in the spring, and complete the experiment in autumn. It would be interesting to follow the whole process, from seeds to rubber.
Ingela Bursjöö, science teacher and science education researcher, Johannebergsskolan Elementary School, Sweden





