Colour to dye for Understand article
The basic chemistry of hair dyes has changed little over the past century, but what do we know about the risks of colouring our hair, and why do we do it?
Every two months Barclay Cunningham goes through a process that begins with taking an antihistamine tablet. After a few hours, she smears a thick layer of antihistamine cream across her forehead, around her ears and over her neck. Finally, she shields the area with ripped-up plastic carrier bags.
All this so she can dye her hair.
It didn’t start out this way. Cunningham coloured her hair for a decade without any problems. Then, one day, she noticed that the skin on her ears was inflamed after she’d dyed her hair. She fashioned plastic bag earmuffs and carried on colouring. But the allergic reaction persisted, so her precautions became more elaborate. Now if she dyes her hair without these measures, she gets an itchy, blistery, pus-filled rash that lasts for weeks.
Suffering for the sake of tinted tresses is not a modern-day phenomenon. Humans have dyed for thousands of years (see box). But the chemical history of modern hair dyes reveals that, while they were once part of an innovative industry, progress has stalled, and today they rely on 125-year-old chemistry.
A long tradition
Archaeological evidence shows that the use of dyes by humans dates back to the Palaeolithic period. Early humans used the iron oxide in soil to decorate their dwellings, textiles and bodies with the colour red. It wasn’t too long before they applied the dyes to their heads.
Ancient Egyptians also dyed their hair, but rarely did so while it was on their heads. They shaved it off, then curled and braided it to wigs to protect their bald heads from the sun. Analysing hair samples has also revealed that the Greeks and Romans used permanent black hair dye. They mixed substances that we know today as lead oxide and calcium hydroxide to create a lead sulfide nanoparticle, which forms when the chemicals interact with sulfur linkages in keratin, a protein in hair. When the direct application of lead proved too toxic, the Romans changed their black dye formula to one made by fermenting leeches for two months in a lead vessel. Not as pleasant as today’s dyes!
The magic of mixing

the future. Model: Jamie
Dietrich
Image courtesy of Kevin
Dooley; image source:
Wikimedia Commons
Understanding the dyes used on hair is not as simple as understanding the colour wheel. We learn in art class that any colour can be obtained by mixing the three primary colours of red, yellow and blue. If you want orange, you mix yellow and red; if you want purple, you combine red and blue; and if you want brown, you mix all three.
Beauticians are taught the same thing when it comes to hair – that brown dye is a combination of three different dyes. “That’s just fictitious information,” says Tom Despenza. Tom has years of experience working in research and development at Clairol. He is now retired and owns his own hair colour company.
Instead, says Tom, “brown hair colour is made up of two chemicals.” Both chemicals are colourless but they produce brown through a chemical reaction that occurs when they’re combined.
Hairdressers are not applying pigments (at least not in the case of permanent hair dye), they are applying a mixture of chemicals to initiate dye formation. The individual molecules have to be linked together before they emit colour, so hair dyes have to sit on the head for 30 minutes to allow this reaction to occur.
A colourful discovery
In the mid-1800s, English chemist William Henry Perkin serendipitously synthesised the first non-natural dye: starting with coal tar, he was hoping to produce the malaria drug quinine but instead created mauve. His discovery revolutionised the textile industry and launched the petrochemical industry. Natural dyes just didn’t have the staying power and vivid colours of the dye that Perkin created. Never before had such a steadfast dye been found.
Soon after, August Hofmann (Perkin’s chemistry professor) noticed that a dye he had derived from coal tar formed a colour when exposed to air. The molecule responsible was para-phenylenediamine, or PPD, the foundation of most permanent hair dyes today.
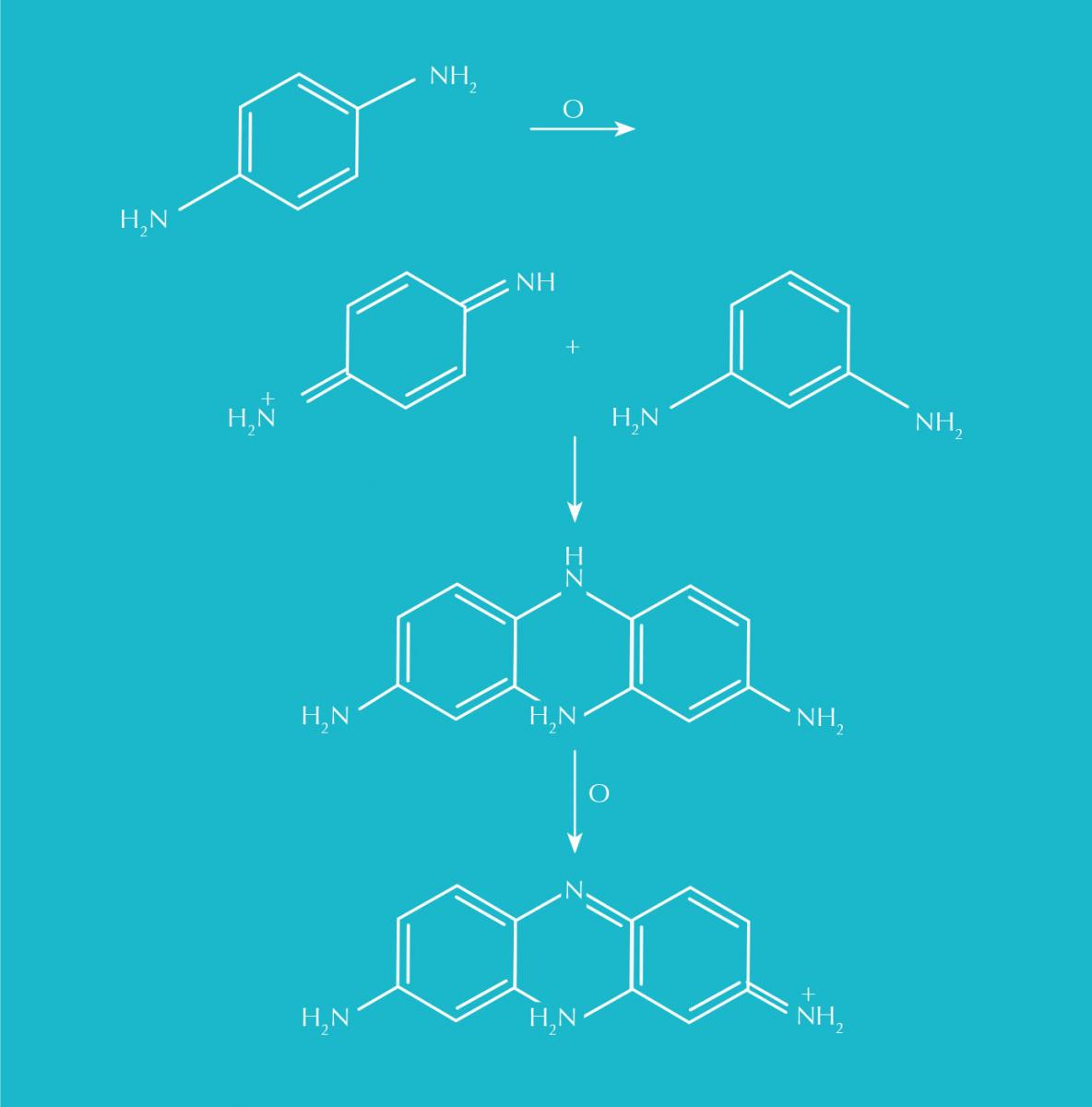
(1,4-diaminobenzene) is oxidised to an imine which then reacts with a coupler.
Another oxidation step then yields a dye
Although hair is a protein fibre, like wool, the dyeing process for textiles cannot be duplicated on the head. To get wool to take a dye, you must boil the wool in an acidic solution for an hour. The equivalent for hair is to bathe it in the chemical ammonia. Ammonia separates the protective protein layers, allowing dye compounds to penetrate the hair shaft and access the underlying pigment, melanin.
Melanin is what gives colour to human skin, eyes and hair. It’s the ratio of two types of melanin – eumelanin and pheomelanin – that determines your natural hair colour. And it’s the size and shape that the melanin molecules form when they cluster in the hair shaft that gives the unique tones within a hair colour. For example, blonds and brunettes have about the same ratio of eumelanin molecules to pheomelanin molecules, but blonds have fewer molecules overall. Naturally blond hair also contains smaller melanin clusters, which reflect light more than the larger clusters found in dark hair.
Along with ammonia, hair dye formulations contain hydrogen peroxide, a bleaching agent. Peroxide serves two purposes: it reacts with the melanin in hair, extinguishing its natural colour, and oxidises PPD molecules to create larger dye molecules. The trapped colour-emitting molecule will remain in the hair, too big to escape.
Early on, dye chemists realised that if they added a secondary molecule, called a coupler, they could manipulate the resulting pigment – a carbon here, a couple of nitrogens there – and multiply the colour choices that were available with PPD alone. Different methods have been proposed, but beauty product manufacturers have yet to accept a permanent hair colour formula without PPD or its related compound p-aminophenol.
Harmful heritage?
For 125 years, the oxidative reaction of PPD has been the extent of hair dye technology. This is “crazy” according to David Lewis, emeritus professor at the University of Leeds in the UK. “Now, I know a lot about dyes and dye stuffs in the textile industry. We would never dream of using this on textiles,” he says. “Primitive, archaic, all these things come to mind. Why do they persist on putting it on human heads?”
Lewis retired from academia ten years ago to launch Green Chemicals, a company that aims to develop safer consumer goods. His company introduced a more environmentally friendly flame retardant, and now Lewis wants to overhaul hair dyes.

Image courtesy of Lisa Creech
Bledsoe; image source: Flickr
One issue is how dyes work: Lewis says that the colour molecules become electron scavengers. This need for electrons is not exclusively fulfilled by other dye molecules, so the electron scavengers also aggressively pursue the skin – causing allergic reactions and potentially damaging DNA.
Globally, haircare products comprise the largest portion of the beauty industry and secure nearly a quarter of the industry’s revenue. In the USA, for example, an estimated 70 per cent of women use hair colouring products.
Reflecting on the heritage of hair dyes, you can’t help but ask: why do so many people still colour their hair? Why would someone go through the rigmarole and tolerate the expense, the itching and the smell? Whatever drives our desire to change the colour of our hair, one thing is certain: people have deep emotional ties to what covers their scalps.
This is clearly true for Barclay Cunningham. At just 12 years old, she began experimenting with her hair and as an adult, she searched for years for the right hair colour. “Never once has it occurred to me to simply not dye my hair,” Barclay says. “The ‘me’ of hair colour happens to come out of a box. The ‘me’ that grew out of my head was not right.”
Acknowledgement
This article is an edited version of an article first published by Mosaicw1, the long form journalism site from the Wellcome Trust.
Web References
- w1 – The full article can be read on the Mosaic website.
Resources
- For a more detailed look at the chemistry behind different coloured hair dyes, Compound Chemistry has created a graphic you can download here.
- The history and synthesis of mauve is outlined for teachers at the Royal Society of Chemistry’s Learn Chemistry site. Download the PDF.
- Before chemically fast dyes were made, people had to rely on natural dyes extracted from plants and animals. Indigo was a purple dye used before mauve was invented, and you can learn how to extract the dye yourself:
- Farusi, G (2012), Indigo: recreating Pharaoh’s dye. Science in School 24: 40–46.
Review
Many scientists are normally seduced by chemistry’s explosions or colour changes. The article shows just how useful this interest can be and why it needs to be encouraged.
The article tells the story of the development of dye – highlighting Perkins’ discovery of mauve and showing how this accidental discovery has allowed the formation of many consumer products.
This quirky tale and history of dyes and colour chemistry could lead to a study of the chemical synthesis of organic dyes and of the effect of using them. A challenge would be to see if school chemists can synthesise dyes that could be used in the art department. Teachers could also use the article to link in with relevant topics in history (history of medicines/cosmetics) and art (methods of textile and fibre dyeing).
Graham Armstrong, Kinross High School, Scotland





