Supporting materials
Download
Download this article as a PDF

With the use of detergents and other surfactants on the rise, the resulting pollution is worrying. One answer: surfactants that can be collected and re-used simply by switching a magnetic field on and off.


It has been estimated that each person in the EU uses about 10 kg of detergents annually. This includes not only the bars of soap, shampoos, toothpastes, washing powders and household cleaning agents we are familiar with, but also detergent-type compounds found in fuels, pharmaceuticals and even foods and beer. In industry, huge quantities of detergents are used, for example in commercial laundries, in preparing cloth and leather for dyeing, in car washes and for cleaning and sanitising hospitals.
Once they have performed their cleaning task, the residues are simply washed away into the sewers and eventually released into the environment. Imagine that instead these residues could be recovered. This article describes some of the latest research into recyclable detergents, and how they can be studied using advanced research methods.
Surfactants are compounds that lower the surface tension of a liquid, making them suitable for a number of applications – such as emulsifiers, foaming agents, wetting agents and dispersants, for example. Surfactants or mixtures of surfactants that are used in cleaning are known as detergents. The simplest and oldest of all detergents is soap, used in Babylon almost 5000 years ago; in fact, soap manufacture is one of the oldest chemical industries.
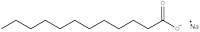
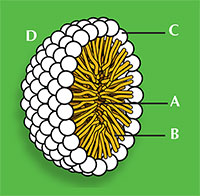
Sodium dodecanoate (figure 1) demonstrates the general structure for all surfactants: one part of the molecule is hydrophilic, meaning it will be soluble in water as it has a charged ‘head’, and the rest of the molecule is an oily hydrophobic ‘tail’. The ability of a detergent to dissolve in water is due to a balance of intermolecular forces. The head is a negatively charged carboxylate ion able to form hydrogen bonds with water, whereas the hydrophobic tail cannot hydrogen bond because it is a long alkane chain, with no electronegative elements present. This explains why surfactants aggregate in clusters, known as micelles (figure 2), which are essential for the action of detergent cleaners.

The two ends of the detergent also behave differently with non-polar stains such as grease. The hydrophobic tails interact with the grease, while the hydrophilic heads attract water molecules through hydrogen bonding. After a little agitation, the grease leaves the material to which it was attached, forming detergent droplets containing grease; the droplet surfaces consist of the water-soluble hydrophilic heads. The grease is therefore removed from the material and held in the water by these micelles (figure 2).
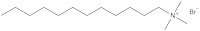
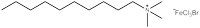
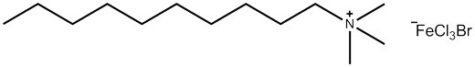
A research group at the University of Bristol, UK – including two of the current authors – is currently working on a new type of surfactant: magnetic surfactants, which respond to a magnetic field as a result of iron atoms in their head groups (figures 3 and 4). These surfactants could have both environmental and medical applications.
How were the magnetic surfactants developed? And how do you prove that you really have produced a magnetic surfactant?
To begin with, the Bristol researchers took a known surfactant and replaced its bromine group with an iron-containing group (figure 3). They then demonstrated that the compound still functioned as a surfactant: it was capable of lowering the surface tension of liquids, and could cause them to foam. Next, the group demonstrated that the iron in the head group had conferred the desired magnetic activity.
To really understand what was happening, however, the researchers needed to look more closely at their compound. For example, although its ability to lower surface tension strongly indicated that it was forming micelles, it was not conclusive proof. To test this, the researchers used a super-sensitive specialist technique called small-angle neutron scattering (SANS).

SANS is an excellent technique for investigating structures about 0.1-100 nm in size; perfect, then, for searching and characterising surfactant micelles and emulsion droplets, which are typically 2-10 nm in diameter. SANS is also widely used to investigate soft matter (e.g. polymers, colloids and liquid crystals), biological molecules (e.g. DNA and proteins in solution) and hard condensed matter (e.g. clusters in alloys, and flux line lattices in supra-conductors).
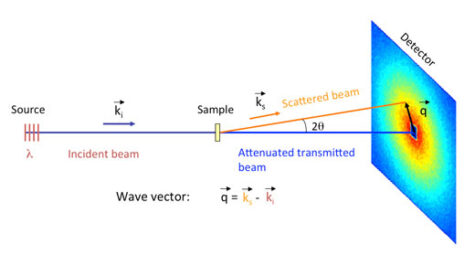
In a SANS experiment, an intense neutron beam is directed onto the sample of interest (figure 5); this beam can be viewed as a stream of free particles travelling in the same direction and with the same speed. The free neutrons in the beam interact with the bound nuclei of the atoms in the sample, scattering the beam. These scattered neutrons are recorded by a position-sensitive detector. The resulting data – the intensity of the neutrons scattered by different areas of the sample – is used in mathematical models to determine the shape, size and charge of the scattering objects in the sample. For a more detailed explanation of SANS analysis, download the supporting materials from the Science in School websitew1.
For their SANS analysis, the Bristol group teamed up with scientists at the Institut Laue-Langevin (ILL; see box) in Grenoble, France. Although other surfactant properties such as surface tension reduction had already been observed with the new compound, the SANS results provided the first conclusive proof that it really was forming micelles.
Furthermore, the scientists were able to show that the micelles formed by the new surfactant were small, spherical and uncharged. This was important, because the behaviour of a surfactant, and thus its applications, are affected by characteristics of the micelles and emulsion droplets that it forms with different liquids. With this information, the scientists are now better able to predict – and in future investigate – the behaviour of their surfactant under different conditions.
Using SANS, the team was also able to test whether in solution, the iron particles dissociated from the surfactant, effectively resulting in a mixture of non-magnetic surfactants and dissolved magnetic particles, or whether the two elements remain bound, forming genuinely magnetic micelles. The results suggested that the iron compounds were firmly integrated in the micelles. This opened up the possibility of creating magnetic emulsions with potential medical applications (see below).

Magnetic surfactants may sound like a strange idea, but they have some very practical applications. For example, many surfactants are not biodegradable. If magnetic surfactants were used instead, they could be retrieved from waste-water using a magnetic field and recycled, resulting in lower levels of detergents entering the environment.

Moreover, currently when there is an oil spill at sea, surfactants are used to break oil slicks into emulsion droplets so small that they diffuse away into the ocean, where the oil remains a pollution hazard. If magnetic surfactants were used instead, the resulting emulsions could be collected, removing both the oil and the surfactants from the water.
Magnetic surfactants may even have medical applications. Targeted drug delivery aims to get medication only to the specific cells where it is required, preventing the waste of valuable pharmaceutical compounds and minimising side effects. Figure 6 shows how emulsion droplets (dyed blue) formed from the magnetic surfactants can be manipulated with a small magnet efficiently enough to overcome typical blood flow in the body. These emulsions could encase medication in the same way as emulsions currently used in drug delivery, but the magnetic emulsions could be directed to the right spot in the body with a magnet.

Magnetic surfactants are not the only new type of surfactants that are currently being developed in Bristol. The research team are also investigating surfactants that can be turned on and off by changes to light, pH, temperature, pressure and carbon dioxide concentration. The current challenge is to learn how to scale up the synthesis of these surfactants to make these smart surfactants cheaply and effectively.

SANS experiments require intense beams of neutrons, which can only be produced at large facilities. In Europe these are done at laboratories jointly funded and run through inter-governmental collaborations, such as ISISw2, in the UK, and the Institut Laue-Langevin (ILL)w3, in France.

ILL is an international research centre operating the most intense steady neutron source in the world. Every year, more than 800 experiments are performed by about 2000 scientists coming from all over the world. Research focuses on science in a variety of fields: condensed matter physics, chemistry, biology, nuclear physics and materials science.
ILL is a member of EIROforumw4, the publisher of Science in School.
Download this article as a PDF