Supporting materials
Download
Download this article as a PDF

Angelika Börsch-Haubold demonstrates the olfactory delights of organic chemistry.

“Chemistry is all bangs and smells.” Not all students of chemistry are drawn to the stenches and explosions in the laboratory. Fortunately, there are many organic molecules that actually smell nice. We experience these substances daily – using a perfumed shower gel, flavouring our meals with herbs, or burying our nose deep in a rose. Teaching the organic chemistry of natural scents should motivate even those who despise the toxic and dangerous side of chemistry to look at structural formulae and functionalities.
In order to be perceptible by our noses, chemicals need to be lipophilic, small (molecular weight < 300 Da) and volatile. Fragrant molecules escape from their fluid or even solid state into the air. The sensory tissue, called olfactory epithelium, is a mucous membrane which lies on the roof of the nasal cavity. Odorants reach this area (approximately 7 cm away from the nostrils) in the air we breathe; if something smells very faintly, we sniff two or three more times, forcing more air and fragrance towards the sensory membrane. There, the molecules dissolve in the mucous and then couple to smell receptors that are expressed on the plasma membrane of sensory cells. The cells send nerve impulses to our brain, which learns to associate the smell with the original substance (such as a rose), lets us recognise it even when the object is hidden (such as when entering a house and knowing that a cake is in the oven) or classifies it as unknown (such as when we go to an exotic restaurant for the first time).
Arranging smells into a limited number of distinct classes is not as easy as defining the basic tastes (sweet, sour, salty, and bitter). Typical smell attributes are flowery (jasmine), spicy (ginger, pepper), fruity (ethyl acetate), resinous (resin smoke), foul (rotten egg), and burning (tar). Musk (muscone), camphor, rancid (isovaleric acid, butyric acid) and pungent (formic acid, acetic acid) are often added to this list. By concentrating more on chemical details, functional groups of fragrant molecules can be linked to characteristic odours (Table 1). The smells of n-aliphatic alcohols, for example, range from herbal, rose and woody to orange. By contrast, n-aliphatic acids smell fatty, sour, rancid or sweaty. Fruit scents are esters composed of short aliphatic organic acids with alcohols. Subtle differences in the chemical composition lead to distinct scents, such as the pineapple aroma of ethyl butyrate and the apricot aroma of pentyl butyrate. Vegetable smells often depend on organosulphur compounds. A ring structure with nitrogen might smell of roasted or fermented foodstuff, whereas aromatic alcohols (phenols) are components of smoked food.
Most of the examples in Table 1 are chosen from different types of food to make the odours easier to demonstrate. You could randomly distribute appropriate plant and food samples on a large surface, and ask students to group the exhibits according to their smell (you could help by providing the attributes ‘floral’, ‘rancid’, ‘fruit’, ‘vegetable’, ‘spicy’ and ‘smoke’). When the students are done, let them compare their results with the chemical classification given in Table 1.
Bearing the structural characteristics in mind, the next question is how we use smell to distinguish between these differences in functional groups, sizes and overall shapes of molecules. In many physiological processes taking place at cell membranes, a receptor is activated after a ligand binds to its extracellular domain. This principle is often depicted as a key fitting into a lock – only the right key opens the corresponding lock – as the docking ligand has high specificity for its acceptor site on the receptor.
| Functional group | Source | Example | Smell |
|---|---|---|---|
| Alcohol -OH |
Plants | Geraniol, linalool Menthol Aroma-active alcohols > c3 |
Fresh, floral Mint Sweet or pungent |
| Aldehyde; ketone -CHO; >C=O |
Fat Milk products |
Diacetyl | Like butter |
| Acid (C1-C12) -COOH |
Cheese | Formic acid Capric acid |
Pungent Like goats’ milk |
| Ester, lactone -COOR |
Solvent (these chemicals are used as solvents) Fruit |
Ethyl acetate Methyl/ethyl butyrate Amyl/butyl acetate Pentyl butyrate |
Glue Pineapple Banana Apricot |
| Pyrazine aromatic =N- |
Roasted, cooked, fermented foods | 2-isobutyl-3-methoxypyrazine 2-acetyl-tetrahydro-pyridine |
Earth, spice, green pepper Popcorn |
| S-compounds: aliphatic, aromatic | Vegetables | Diallyldisulphide 1,2-dithiolane-4-carboxylic acid |
Garlic Asparagus |
| Phenols (mono-, poly-) | Smoked food | Guaiacol Cresol |
Wood smoke Tarry |

Some of the most fascinating features of the physiology of smell were discovered by the 2004 Nobel Prize winners Linda Buck and Richard Axel. In contrast to the simple but specific key-lock model that governs taste, smell is dictated by a whole set of sensory cells. One type of fragrant molecule interacts with more than one receptor type, so the overall sensation is created by the combination of activated receptors. When they tested a series of aliphatic n-alcohols on individual mouse neurons, Buck and co-workers found that clusters of olfactory neurons were activated. For example, pentanol weakly stimulated a receptor called S3; hexanol strongly activated S3 and S25; heptanol S3, S19 and S25; octanol S18, S19, S41 and S51; and nonanol S18, S19, S41, S51 and S83. Thus, a single odorant is recognised specifically by multiple odorant receptors working in combination.
Such a pattern of receptor activation creates a vast repertoire of perceivable odours. Indeed, it is estimated that we can remember about one thousand odours and distinguish between an order of magnitude more, depending on our age, experience and natural sensitivity. Our linguistic ability to name scents, however, lags far behind our nose’s ability to discern them. The food industry trains experts to recognise subtle nuances of smells. For example, diluted essential oils or isolated compounds representing a certain aroma are applied onto a strip of odourless filter paper and placed into a screw-top glass vial.

An untrained person may correctly associate an odorant with its source or something that contains it, but still fail to identify the original substance. In a course on the sensory evaluation of food that I recently taught, only half of the students recognised the aroma of oregano; a quarter of them confused it with thyme or marjoram, which are closely related herbs; and others identified the scent as rosemary, basil and parsley, which are less closely related herbs with quite distinct flavours. However, some students correctly associated the odour with tomato soup, which may be flavoured with oregano. Two-thirds of the class recognised the smell of roses, some students associated it correctly with similar flowers such as lilac or melissa or with perfume, but others incorrectly suggested mint, medicine and spice.
Almost all students recognised bitter almonds, mint and cinnamon, but failed to identify sage and coriander. When the solutions were revealed, everybody immediately recognised the smells that had previously eluded them, demonstrating that olfactory memory is far better than our ability to actually name a particular smell.
The fine-tuning of our receptor machinery allows us to distinguish between chemically similar molecules. Many fragrances are derived from plants, and plant products can be used to demonstrate how small changes in the chemical structure of an odorant give rise to either completely different smells or at least distinguishable flavours (see illustrated structures). The examples are sorted by the following chemical principles:
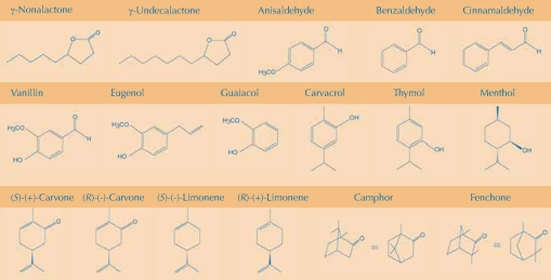
However, there are compounds that are structurally unrelated but smell similar. Cyclooctane, camphor and 1,8-cineol all have a camphoric odour, although cyclooctane consists of an all-carbon single ring structure (C8H16) whereas camphor and 1,8-cineol are both bicyclic molecules with a functional group containing oxygen. The so-called ‘green note’ of unripe fruit and vegetables, which is added to cosmetics for a scent of ‘freshness’, comes from a group of closely related C6 aldehyde compounds (cis-3-hexenal: fresh tomatoes; cis-2-hexenal: green apples; trans-2-hexenal: green and black tea, fresh tomatoes). Similarly, the alcohol cis-3-hexenol (freshly cut grass), the C9 aldehyde 2-trans-6-cis-nonadienal (cucumber peel), an aldehyde attached to a ring structure (the grassy-smelling ligustral) and even some pyrazines (2-propyl-3-methoxypyrazine: bell peppers) have this ‘green note’.

By using the wide range of odorants contained in plants, you can combine organic chemistry with the physiology of smell to demonstrate how well we understand our environment without being fully aware of it. If you want to focus on training the sense of smell, you could ask your students to sort different dilutions of one odorant by increasing intensity (see Experiment 2 in the classroom activityw2). These two activitiesw1, w2 should stimulate students to link everyday situations with chemistry and make them curious about the description of our world in terms of chemical compounds.
I would like to thank the students from the sensory training course (Fachhochschule Weihenstephan, Winter semester 2006/07) for the preparation of odorant samples and for stimulating discussion.
Students may be pleasantly surprised to discover that chemicals not only are responsible for potentially dangerous and/or unpleasant effects but also give rise to delightful odours and tastes. The activities that Angelika Börsch-Haubold presents here may be used to demonstrate that the realm of smell resides in chemistry and that our noses are exquisitely sensitive chemical sensors, able to detect subtle variations in molecular structure.
The article is applicable to organic chemistry in general, with specific relevance to food technology. It could be linked to a discussion of the use of (unnatural) food additives or of genetic modification of food crops. It would also be useful background reading for teaching lessons on the senses.
Matthew Fletcher, UK
Download this article as a PDF