Oxyntomodulin: a new therapy for obesity? Understand article
Katie Wynne and Steve Bloom from Imperial College London, UK, describe their work on a hormone that could tackle the causes of obesity.
Click on image to enlarge.
Image courtesy of Katie Wynne
The obesity epidemic has overtaken Europe. It is estimated that 200 million of the 350 million adults living in the EU are overweight or obese. In fact, the proportion of overweight and obese men is even higher in some European countries than it is in the USA. Of greatest concern is the fact that the number of overweight children in the EU is increasing by 400 000 every year, a warning that the obesity problem will be far worse in the future.
Obesity increases the risk of heart disease, diabetes, stroke, respiratory disease, arthritis and cancer. This disease burden has a disastrous effect on health and costs 7% of the total EU health-care spending. The risk of developing obesity-related conditions can be reduced by losing as little as 5% of body weight. However, public-health campaigns to promote healthy eating and exercise have not stopped Europeans becoming progressively fatter. Currently available drug treatments in combination with diet produce only modest short-term weight loss and are limited by side-effects. Major gastro-intestinal surgery for obesity can result in permanent weight reduction, but the frequency of severe post-operative complications limits its use to extreme cases. Research groups worldwide are urgently searching for an effective anti-obesity treatment. The Department of Metabolic Medicine at Imperial College London has led the field in appetite research and has recently published exciting work regarding the role of the gut hormone oxyntomodulin as a novel therapy for obesity.
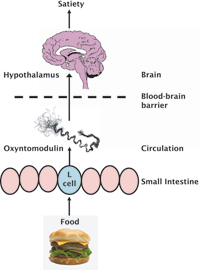
Oxyntomodulin is one of a group of gut hormones: small molecules released from the gastrointestinal tract with several physiological actions including effects on digestion and appetite. Oxyntomodulin was identified over two decades ago, when it was found to reduce stomach acid and delay emptying of stomach contents in rodents. However, the available data showed no effect on gastric emptying in humans and its role in the control of appetite has only recently emerged. Oxyntomodulin is released from the small intestine after each meal in proportion to the calories eaten. Early studies showed that administration of synthetic oxyntomodulin reduces appetite, suggesting that natural oxyntomodulin signals to the brain a feeling of ‘fullness’ or ‘satiety’ after eating (see below).
Click on image to enlarge.
Image courtesy of Katie Wy
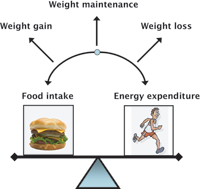
This research stimulated interest in developing the peptide as a possible anti-obesity therapy, harnessing the body’s own satiety signal in order to reduce food intake. Body weight is maintained by the balance between food intake and energy used, or ‘energy expenditure’ (see right). Total energy expenditure can be divided into the energy used by the body for metabolic processes at rest and that used during activity. Obesity occurs when overeating and low levels of physical activity tip the balance in favour of weight gain. We have recently performed studies in healthy overweight and obese volunteers to investigate the effect of oxyntomodulin on food intake, energy expenditure and body weight.
A double-blind, placebo-controlled study was performed in which 15 healthy overweight and obese volunteers were trained to give themselves oxyntomodulin injections under the skin, just before each meal, three times daily. Food intake and energy expenditure were measured over four days and compared with a similar period during which the same volunteers administered a saline placebo. During the study, the volunteers and investigators were unaware of the identity of the administered substance. Food intake was recorded at a study meal in which food was provided in excess and the volunteers ate until they felt full. Activity-related energy expenditure was calculated from combined heart rate and movement monitoring in the participant’s normal environment using a new biosensor. Resting energy expenditure was calculated using indirect calorimetry, which measures the amounts of oxygen consumed and carbon dioxide produced in each breath by the volunteer.
Clinical drug trials
New drugs must go through a series of trials, known as phases, in order to test whether they are effective and safe.
PHASE I: Early trial in a small number of usually healthy volunteers to establish a safe dose and look for potential side-effects
PHASE II: Larger group trial of volunteers (up to 100) with the illness to be treated, to establish short-term effectiveness and safety. Both studies described in this article were early Phase II trials.
PHASE III: Large group drug trial in volunteers (up to several thousand) with the illness, over an extended period of a year or more to compare the treatment with an existing therapy or a placebo.
PHASE IV: Drug trial usually performed after a treatment has been licensed, to establish the effectiveness of the treatment when it is used more widely and to investigate long-term risks and benefits.
This process is essential to ensure that the benefit of the treatment is greater than any possible side-effects, but it means that it can take several years for a new drug to reach the public.
Potential obesity drugs entering each phase of clinical trials between 1994-2007. Although many potential drugs are investigated, few reach the market: only two are currently licensed in the USA.
| Phase | I | II | III | IV |
|---|---|---|---|---|
| Number | 124 | 259 | 169 | 55 |
treatment. The error bars
represent standard error of
the mean. Any difference
marked with an asterisk is
statistically significant: one
asterisk represents a
significant difference when p
< 0.05; two asterisks r
epresent a significant
difference when p < 0.01.
Click on image to enlarge.
Image courtesy of Katie Wynne
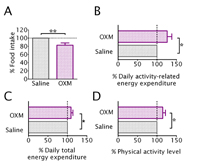
The results of this study showed that after an injection of oxyntomodulin, volunteers ate on average 128 Kcal or 17.3% less (see below, A) without altering their enjoyment of food. In addition, the participants’ energy expenditure due to activity was markedly increased by 143 Kcal/day or 26.2% during the period of oxyntomodulin treatment (see below, B). The increase in activity resulted in an increase in total energy expenditure of 9.4% (see below, C), although resting energy expenditure was unchanged. These overweight and obese people started with expected low levels of physical activity, but oxyntomodulin administration increased physical activity back toward normal levels (see below, D), resulting in more energy being used each day. These findings suggest that oxyntomodulin could be an ideal intervention for the obese, as it has a double effect of suppressing appetite and concurrently increasing physical activity toward normal
The results of this study showed that after an injection of oxyntomodulin, volunteers ate on average 128 Kcal or 17.3% less (see below, A) without altering their enjoyment of food. In addition, the participants’ energy expenditure due to activity was markedly increased by 143 Kcal/day or 26.2% during the period of oxyntomodulin treatment (see below, B). The increase in activity resulted in an increase in total energy expenditure of 9.4% (see below, C), although resting energy expenditure was unchanged. These overweight and obese people started with expected low levels of physical activity, but oxyntomodulin administration increased physical activity back toward normal levels (see below, D), resulting in more energy being used each day. These findings suggest that oxyntomodulin could be an ideal intervention for the obese, as it has a double effect of suppressing appetite and concurrently increasing physical activity toward normal
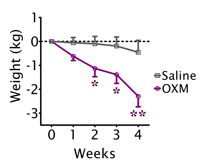
The obesity crisis is fuelled by the easy availability of highly palatable, calorie-dense food and a lack of physical activity. Normal dieting tends to reduce the amount of energy each individual uses, making it particularly difficult for obese people to lose weight. Oxyntomodulin, by contrast, reduces food intake and also increases energy usage back toward normal levels, an effect thought to be mediated via the hypothalamus (see box). Appetite suppression is well maintained over a four-week period. During this time, we observed considerable weight loss in the obese subjects accompanied by changes consistent with a loss of body fat.
groups. The error bars represent
standard error of the mean. Any
difference marked with an asterisk
is statistically significant: one asterisk
represents a significant difference
when p < 0.05; two asterisks represent
a significant difference when p < 0.01.
Image courtesy of Katie Wynne
Currently, the only successful treatment for obesity is surgery, but its use is limited by the dangers of the operation and subsequent side-effects.
Surgery which causes food to bypass part of the intestinal tract alters the levels of circulating gut hormones, including increasing the levels of oxyntomodulin, which results in a loss of appetite. The successful weight loss observed after surgically induced modulation of oxyntomodulin suggests that drug treatment with oxyntomodulin over many years may be effective without the complications that restrict the use of surgery.
Large clinical trials are now needed to confirm the therapeutic potential of oxyntomodulin over a longer period. These Phase III trials (see box) would involve testing the effectiveness of the hormone in hundreds and thousands of volunteers over a period of several years. This large-scale research is needed to be certain that the treatment will be effective and to look carefully for any uncommon side-effects that may not have been noted in smaller studies. Using the body’s own method of controlling weight may be a viable method of treating obesity without the serious side-effects caused by currently licensed drugs. In the future, this could provide a new way to tackle the obesity epidemic, with oxyntomodulin providing an effective weapon.
The hypothalamus
Oxyntomodulin acts on the hypothalamus, an area of the brain known to be important for the control of energy balance. Oxyntomodulin and other circulating signals are able to cross the barrier between the blood and the brain, via a specialised area next to the hypothalamus. The hypothalamus can then receive and integrate these signals. Neurotransmitters are released, which send messages from the hypothalamus to many different brain regions and result in changes in behaviour. Thus, an increase in the circulating levels of oxyntomodulin results in a feeling of satiety and allows an increase of activity levels. This may be a logical combination of effects, as it would allow a period of intense activity to occur when there is enough food to supply energy.
References
- The research described here was published as:
- Wynne K et al. (2005) Subcutaneous Oxyntomodulin Reduces Body Weight in Overweight and Obese Subjects: A Double-Blind, Randomized, Controlled Trial. Diabetes 54: 2390-2395
- Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, Bloom SR (2006) Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. International Journal of Obesity 30: 1729-1736. doi:10.1038/sj.ijo.0803344
- Department of Metabolic Medicine, Imperial College London website
- International Obesity Task Force Report on Obesity: www.iotf.org/aboutobesity.asp
- International Obesity Task Force Report on Childhood Obesity: www.iotf.org/childhoodobesity.asp
- The Wellcome Trust’s Big Picture series provides teachers and students (aged 16 and above) with up-to-date information on research findings in biomedicine, and the social and ethical implications of this research. These resources can be downloaded or ordered online. A recent issue of the Big Picture focused on obesity: www.wellcome.ac.uk/node5951.html
Review
Obesity, diets and healthy eating habits are of high interest to teenage and young adult students. This article provides solid scientific data on the central brain control of food intake and physical activity, and opens many new topics for discussion:
- The control of protein synthesis and possible malfunctions within gut cells
- The circulation of oxyntomodulin and its feedback to the release of neurotransmitters via the hypothalamus, and the information exchange between brain and body.
- The hypothesis that a feeling of satiety can lead to higher physical activity
- The effects on hormone metabolism – both positive and negative – of injecting hormones
- Current interest in anorexic and bulimic eating disorders, and the complexity of balancing body weight.
In addition, this article is an excellent example of scientific and medical research procedures for searching for cures for everyday health risks. The contents can be easily adjusted for middle-school students, whose interest will lie primarily in weight gain or weight loss. Older students will be challenged by the brain-body interactions of food intake and physical activity, as well as by cellular control mechanisms of physiological functions. The detailed description of clinical drug-trial procedures can be used to enrich cutting-edge biology teaching.
Friedlinde Krotscheck, Germany





