Two hydrolytic enzymes and an epistemological–historical approach Teach article
Why are enzymes so special? How do they differ from inorganic catalysts? Isabella Marini from the University of Pisa, Italy, describes a classroom protocol to enable students to answer these questions for themselves.

and her students at the Liceo
Scientifico Ulisse Dini, Pisa
Image courtesy of Isabella
Marini
Can history and epistemology help us to teach science?
When students begin to learn about chemical reactions, they reason as alchemists, not as chemists: when they see a chemical reaction, they think in terms of transmutation (as alchemists did) and not of transformation (as the modern chemist does). As they learn, students re-run the development of human knowledge; this is a critical process that cannot be underestimated.
Although teachers need not necessarily teach specific lessons on the history of science, they must be aware of its importance. Science history and epistemology (the philosophy of knowledge) are fundamental because they, together with psychology, help us to understand what is required at each phase of the educational process. Recognising that humans have observed particular phenomena and have misinterpreted them for hundreds of years, and that the solution to these complex problems forms the basis of science, helps us to realise that many arguments are incomprehensible to our students without a gradual approach to new concepts.
When introducing the idea of enzymes to younger secondary school students, I wanted the students to discover for themselves what makes enzymes so special and so important. I chose amylase and invertase, two accessible enzymes whose catalytic activity can be easily detected without any instruments except the eyes.
Some historical hints
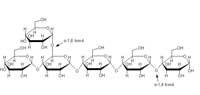
of starch. Amylase catalyses
the endohydrolysis of α(1-4)
glucosidic bonds. Click to
enlarge image
Image courtesy of Isabella
Marini
In 1812, Kirchhoff succeeded in hydrolysing starch by heating it with sulphuric acid. Surprisingly, the pH did not vary, suggesting that the acid did not take part in the reaction; however, its presence was indispensable.
Two decades later, Payen and Persoz performed an ethanol precipitation and isolated a white, water-soluble substance from germinating barley (Payen & Persoz, 1833). This substance, capable of hydrolysing starch, was named diastase. Diastase was later renamed amylase, but the suffix ‘-ase’ has remained to indicate almost all the enzymes we know.
In 1835, Berzelius demonstrated that the germinating barley extract catalysed starch hydrolysis more efficiently than did sulphuric acid. He coined the term ‘catalysis’; very small amounts of a catalyst increased the rate of a particular reaction without being used up. The rates of biochemical reactions could thus be explained; special catalysts in cells were able to operate in mild conditions. The same idea underlies these experiments.
Amylases
To use the carbon and energy stored in starch, the human digestive system must first break the polymer down into smaller sugars. Salivary α-amylase (1,4-α-D-glucan glucanohydrolase; Enzyme Commission (EC) number 3.2.1.1) begins the digestion of polysaccharides in the mouth (the process is completed in the small intestine by the pancreatic amylase). It is a monomeric calcium-binding glycoprotein which randomly hydrolyses the α(1,4) glucosidic bonds in starch (see diagram).
β-amylase (1,4-α-D-glucan maltohydrolase, EC 3.2.1.2) catalyses the hydrolysis of the α(1,4) glucosidic bonds in starch, removing successive maltose units from the non-reducing ends of the chains. It is one of the major proteins found in the starchy endosperm of barley (Hordeum vulgare) grain and it is a key enzyme in the degradation of starch during brewing.
Invertase
Invertase or sucrase (sucrose-α-D-glucohydrolase; EC 3.2.1.48) catalyses the hydrolysis of sucrose and maltose. Sucrose, commonly known as cane sugar, is a disaccharide composed of an α-D-glucose molecule and a β-D-fructose molecule linked by an α1-β2 glycosidic bond. When this bond is hydrolysed, an equimolar mixture of glucose and fructose is generated (see diagram). In yeast (Saccharomyces cerevisiae), invertase exists in intracellular and extracellular forms.
Classroom protocol
Materials
- Phosphate buffer, 50 mM, pH 7 (Buffer A). This is prepared by dissolving 3.55 g dibasic phosphate (Na2HPO4) in distilled water and titrating to pH 7.0 (with a pH meter) with hydrochloric acid (HCl) before adding distilled water to make the final volume up to 500 mL.
- Solutions in Buffer A: starch 10 g/L, sucrose 0.1 M, glucose 0.1 M and fructose 0.1 M.
- Iodine solution N/50. Distilled water is added to 20 g KI and 12.7 g iodine, to make the volume up to 1 L. This solution is diluted 1:5 with distilled water.
- Fehling solutions A (7 g CuSO4 in 100 mL distilled water) and B (34 g potassium sodium tartrate and 12 g NaOH in 100 mL distilled water).
- 5 M sodium hydroxide (NaOH)
- 5 M hydrochloric acid (HCl)
- Saliva. In saliva, α-amylase is already in solution and does not require homogenisation. It can simply be diluted 1:10 with Buffer A.
- Barley seeds. Homogenise germinated barley seeds (three to five days after sowing) by grinding them in a mortar and pestle with Buffer A (about 1 g seeds/mL buffer). Centrifuge the extract at 15,000 g for 5 min; the liquid is used as the β-amylase sample. If you do not have access to a centrifuge, filter the mixture and use the filtrate.
- Yeast solution 0.4 g/L in Buffer A (baker’s yeast, which can be bought from the supermarket).
Methods
Iodine method
Iodine in aqueous solution with starch forms a blue-violet complex of high sensitivity and specificity. Maltose and glucose are colourless in the presence of iodine.
Fehling method
When heated in the presence of reducing sugars (such as glucose or fructose, but not sucrose or starch), an alkaline solution of cupric ions (Cu2+) is reduced to cuprous ions (Cu2+), forming a yellow-red precipitate of cuprous oxide (Cu2O).
Procedures
To demonstrate the test methods, test all four carbohydrate solutions (starch, sucrose, glucose and fructose) with both the Fehling and iodine methods. Starch is the substrate of amylase; sucrose is the substrate of invertase, whereas glucose and fructose are the products of the reaction it catalyses.
Amylases
Starch hydrolysis is shown either by the disappearance of the blue colour in the presence of the iodine solution or by a yellow-red precipitate with the Fehling test.
For both α-amylase (saliva) and β-amylase (barley extract), prepare seven test tubes, mixing 2 mL Buffer A with 400 µL starch solution. Then treat each tube as described in Table 1.
| Tube | HCl | Salaiva Or Barley Extract | Heat on the Bunsen burner for: | Leave at room temperature for: |
|---|---|---|---|---|
| 1* | 2 drops | 5 min | ||
| 2* | 2 drops | 5 min | ||
| 3 | 5 min | |||
| 4 | 5 min | |||
| 5 | 0.5 mL | 5 min | ||
| 6* | 2 drops | 0.5 mL | 5 min | |
| 7 | 0.5 mL** | 5 min |
Divide the contents of each test tube into two parts: test one part with 2 drops of iodine, the other with 500 µL Fehling A and 500 µL Fehling B solution. Enter the results of the tests in Table 3.
To test amylase specificity, repeat the procedure for tube 5, replacing saliva with 300 µL yeast solution (yeast does not contain amylases) in tube 5.
Invertase
Prepare seven test tubes, mixing 1 mL Buffer A and 0.5 mL sucrose solution. Then treat each tube as indicated in Table 2.
| Tubes | HCl | Yeast Extract | Heat on the Bunsen burner for: | Leave at room temperature for: |
|---|---|---|---|---|
| 1* | 1 drop | 5 min | ||
| 2* | 1 drop | 5 min | ||
| 3 | 5 min | |||
| 4 | 5 min | |||
| 5 | 1 mL | 5 min | ||
| 6* | 1 drop | 1 mL | 5 min | |
| 7 | 1 mL** | 5 min |
Test each tube with 500 µL Fehling A and 500 µL Fehling B.
Enter the results of the tests in Table 3.
| Tube | α-amylase (saliva) | β-amylase (barley extract) | Invertase | ||
|---|---|---|---|---|---|
| Iodine test | Fehling test | Iodine test | Fehling test | Fehling test | |
| 1 | |||||
| 2 | |||||
| 3 | |||||
| 4 | |||||
| 5 | |||||
| 6 | |||||
| 7 | |||||
To test invertase specificity, repeat the procedure for tube 5, replacing the yeast solution with 500 µL 1:10 diluted saliva.
Safety
These experiments do not use or generate any hazardous reagent, except for HCl or NaOH. These reagents and saliva should only be handled while wearing safety gloves.
Iodine must be weighed carefully and a safety mask should be worn, as iodine is easily sublimated. Care must be taken when heating tubes with the Bunsen burner and while dissolving starch in the warm buffer.
Expected results
Together, the extreme conditions resulting from heating and the addition of hydrochloric acid enable starch and sucrose to be hydrolysed. However, neither heating nor hydrochloric acid addition alone is effective.
Under mild conditions (no heating and no addition of acid), saliva, barley and yeast extracts hydrolyse their substrates. Both heating and the addition of hydrochloric acid prevent the hydrolysis: the extracts are thermolabile and sensitive to pH.
Discussion
Comparing the extreme conditions required for chemical catalysis (elevated temperature and extreme pH) with the mild conditions required by biological extracts introduces students to the idea of a ‘special and powerful substance’ present in living organisms, which is thermolabile, specific (unlike inorganic catalysts) and able to catalyse reactions. In this way, high-school students can re-run the development of historical knowledge about enzymes; the term enzyme will no longer seem obscure because “words can detach and preserve a meaning only when the meaning has been first involved in our own direct intercourse with things” (Dewey, 1910).
Acknowledgments
I wish to thank Professor Umberto Mura, Department of Physiology and Biochemistry, University of Pisa, for his constant encouragement. Special thanks are due to Professor Rosanna Striccoli for the English revision of the manuscript and also to my students for their precious observations and doubts.
References
- Dewey J (1910) How we think. Boston, MA, USA: D.C. Heath & Co
- Payen A, Persoz JF (1833) Mémoire sur la diastase, les principaux produits de ses reactions et leur applications aux arts industriels. Annales de chimie et de physique 53: 73-92
Resources
- Marini I (2005) Discovering an accessible enzyme: salivary α-amylase. Prima digestio fit in ore: a didactic approach for high school students. Biochemistry and Molecular Biology Education 33: 112-116. doi:10.1002/bmb.2005.494033022439
- Inhelder B, Piaget J (1958) The Growth of Logical Thinking from Childhood to Adolescence. New York, NY, USA: Basic Books
- Voet D, Voet JG (2004) Biochemistry, 3rd Edition. Hoboken, NJ, USA: J. Wiley & Sons
- van der Maarel MJ et al. (2002) Properties and applications of starch-converting enzymes of the alpha-amylase family. Journal of Biotechnology 94: 137-155. doi:10.1016/S0168-1656(01)00407-2





