Supporting materials
issue12_nature_abrahamson2000.pdf
Download
Download this article as a PDF

Halina Stanley describes how two Israeli scientists investigated plasma balls and in the process found a potentially useful way to create nanoparticles.

There are things we all know we shouldn’t do, but – whether by accident, through curiosity, or simply because we know we shouldn’t do them – we do them anyway. Not putting metal objects in a microwave oven is probably rule number one of microwaving – and we all know why, because we’ve all at some time left a fork on the plate of leftovers to be reheated, creating arcs, sparks and perhaps plasma balls (commonly called fireballs – the two terms are used interchangeably in this article) before we hurriedly hit the ‘off’ button. Judging by the number of wacky and downright dangerous experiments you can find videoed on YouTubew1, many young (and not so young) people find creating fireballs irresistible.
Scientists from Tel Aviv University in Israel have now deliberately created fireballs in a microwave cavity at the European Synchrotron Radiation Facility (ESRF) in Grenoble, France: they wanted to find out what was in them. Their results show that highly ionised nanoparticles (a dusty plasma) can be made in your school’s microwave oven.
The scientists, Eli Jerby and Vladimir Dikhtyar, were interested in using microwaves to produce strong localised heating. In fact, they were deliberately introducing a metal electrode to focus microwaves on a point only a few millimetres across (exactly the opposite of what you want to do when heating food). If you heat materials like glass or ceramics, the amount of microwave energy that they absorb increases as they become hotter (the dielectric constant is very temperature-dependent). Warmer regions absorb more microwaves and heat up more. This positive feedback effect, called thermal runaway, is potentially dangerous, especially in materials that are poor thermal conductors where slow heat exchange with surrounding material means that you can get very, very hot spots (over 1200 °C).
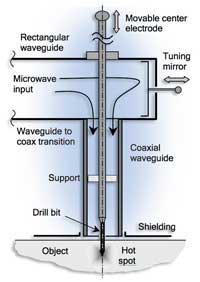
This was exactly what Jerby and Dikhtyar were aiming to produce: they were developing a drill that uses microwaves to make 2 mm diameter holes in ceramics or glass while leaving metallic substrates untouched. (This drill is silent and does not produce dust, and has the potential for drilling bones in orthopaedic surgeryw2).
One day (perhaps inevitably!) something went wrong: a fireball detached itself from the molten material. “It destroyed a component of the microwave generator worth about $2000,” says Professor Jerby. Fireball production must have become quite a problem: “On several occasions a fireball arose out of a hot spot and was blown into the air. The fireball moved like an elastic glowing balloon, floated in the air toward the microwave antenna about 20 cm away and disappeared” (Dikhtyar & Jerby, 2006). Fireballs could appear repeatedly (for example at one-second intervals), but the phenomenon was rare and unpredictable.
Although Jerby and Dikhtyar were not fundamentally interested in fireball production to begin with (just desperate to get rid of it), as time passed, their interest increased. The next few years were devoted to systematic work so that they could intentionally generate fireballs from molten spots of glass. Now, in addition to using their drill to make holes, they can use a modified version of it to make fireballs.
There are some videos of Professor Jerby’s fireballs on his websitew3, but plenty of amateur investigators around the world have performed the experiments, too. No one should even think of repeating the experiment done by Bill Beaty, an engineer at the University of Washington in Seattle, USA (the risk of hot flying glass or a broken microwave seems much too great to me) but his videow4 is too entertaining to miss.

Creating fireballs is one thing. Understanding them is quite another. As you can see on YouTube, people have created fireballs by microwaving burning candles, grapes, bits of aluminiumw5 and smouldering toothpicks as well as molten glass. All these fireballs are buoyant in air and are sustained while being irradiated with microwaves, although they are extinguished shortly after the microwave power is shut off – lasting around 30 ms in the case of molten glass. They have some of the reported characteristics of ball lightningw6, which, according to a proposed model, is caused by ordinary lightning throwing a cloud of nanoparticles out of the soil that slowly oxidise in the air releasing heat and light (Abrahamson & Dinniss, 2000).
Obviously Jerby, Dikhtyar and their colleagues wanted to understand their fireballs. It appeared as if they were drawing material out of the molten glass (see image below and the videos on Professor Jerby’s websitew3), but if there were glowing particles suspended in the air, they had to be really small. If there were particles even as large as a couple of microns the fireballs wouldn’t just disappear (as they do) when the microwaves are turned off – the particles would scatter visible light in the same way as water droplets in a cloud (which have an average size of about 10 ?m) and you would see a cloudy haze of glass droplets.
Electron microscopy is usually the first technique that scientists use to characterise sub-micron structures – such as the hypothesised particles in the plasma balls – but you cannot create a sample of a plasma ball to be put in an electron microscope’s vacuum tube. However, the technique of small-angle scattering (see box) provides a way to tell whether there are particles in a plasma ball, and – if so – to characterise any particles found.
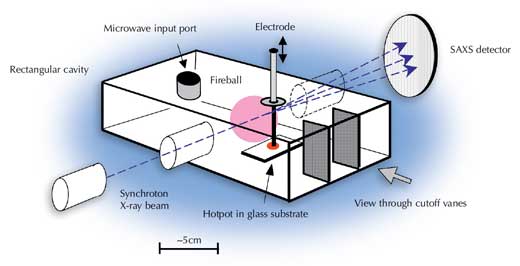
Therefore, Jerby and his colleagues took a microwave cavity containing their fireball-creating mechanism (the modified drill) to ESRF, where very intense beams of X-rays are used to study materials. The microwave cavity had holes to allow the X-rays in and out, and a viewing port, so that the researchers could see what they were doing. The X-ray entry and exit holes were too small to allow microwaves (wavelength ?12 cm from a 2.45 GHz magnetron) to pass through, and the viewing window had vanes to prevent microwaves escaping (see image to the left).
At ESRF, the researchers created fireballs in their cavity using the modified drill. X-rays (wavelength 0.1 nm) were fired from the synchrotron down an evacuated tube, through a cover (transparent to X-rays) and into the microwave cavity filled with air at atmospheric pressure. The X-rays shot through the fireballs (which stayed immobile for about 1 s) and exited the cavity into a second evacuated tube which led to an X-ray detector 5 m away (see diagram). The small-angle X-ray scattering patterns produced were recorded every 0.1 to 0.3 s.
These patterns proved that the fireballs were indeed full of particles with an average radius of about 25 nm – i.e. they are nanoparticles. The data also showed that the particles varied widely in size (as is typical of aerosols) and that there were about 109 particles per cubic centimetre. This makes the volume fraction of solid material (the ratio of volume of solid to total volume of space) in the fireball around 10-7 or 10-8. There was really only a very, very, small amount of matter in the cloud. The analysis also suggested that the particles had quite a rough surface: the scientists found the surface to have a fractal dimension of 2.6 (2.0 corresponds to a smooth 2D surface, 3.0 to 3D).
Small-angle scattering (SAS) is a technique in which light, X-rays or neutrons are fired through a sample and the radiation that is deflected slightly (scattered close to the straight trajectory) is analysed (see diagram). The angle (2θ) by which the radiation deviates from the straight trajectory depends on the wavelength of the incoming radiation and the size of the scatterer (in this case, the particle in the sample being analysed). For a given wavelength radiation (λ), the larger the angle, the smaller the particle, or conversely, the smaller the angle, the larger the length scale (d) being probed. The relationship between these parameters is given by λ/d = 2sin(θ). The visible (laser) light used for light scattering experiments has a much longer wavelength (e.g. 600 nm) than neutrons (about 0.1 – 1 nm) or X-rays (≈ 0.1 nm) and (although there is some overlap) it therefore probes larger objects.
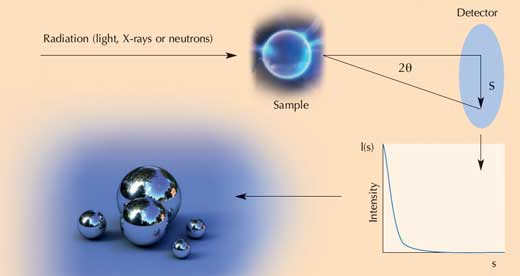
The SAS technique can tell you the average size of a particle (in the range of about 1 nm to a few hundred nanometres), the size distribution of particles, the shape of the particles, and something about their internal structure, surface roughness or the interparticle separation, but not all this information can be extracted at the same time from any given data. The scattering pattern is relatively lacking in sharp features (usually there is just some overall decline in scattered intensity as scattering angle increases, occasionally there may be one broad peak) and does not uniquely define the scatterers: a very similar small-angle scattering pattern may, for example, be produced by a polydisperse (of varying size and shape) population of spherical scatterers or a fairly monodisperse (of same size and shape) population of cylindrical scatterers. Consequently, data analysis proceeds by a ‘guess, check and revise’ method, where a plausible model is used to calculate a predicted scattering pattern, which is then compared with the actual data, and the model is revised accordingly with all steps being repeated iteratively.
But why do the particles glow? Why do the researchers say they form a plasma ball? While the particles are being microwaved they absorb microwave energy and heat up to about 730 °C (1000 K). This energy is re-radiated in the form of intense visible light. At 730 °C the particles will also emit electrons due to thermoionic emission, thus making the fireball a dusty plasma (a cloud of solid particles that have lost electrons and are thus highly ionised).
Using X-rays at ESRF, the scientists also investigated what happens to the fireballs when the microwaves are turned off. Visually the fireball vanishes after about 30 ms, but the X-ray data continued to detect particles for about 4 s. The particles were there, but invisible to our eyes because they were so small. These X-ray data showed that the particles (which were charged and stable while being microwaved) initially simply diffuse away as the fireball cools and then, as cooling continues, tend to aggregate and form large clusters (Mitchell et al, 2008).
Professor Jerby has since returned to ESRF with a collection of different materials to microwave. He says, “We examined the structures of plasma balls made from a variety of materials, including copper, salts, water and carbon. It seems that we are able to generate plasma balls from almost any material now….” This means that he now has a method of directly creating nanoparticles of many different substances. This is very interesting, because nanoparticles are increasingly important in a wide variety of applications, and producing them is not always easy. Nanoparticles are being used in medicine (e.g. drug delivery), in catalysis (for cleaning up pollutants), and even in treatments for smelly socks (which rely on nanoparticles of silver to kill bacteria; see Benn & Westerhoff, 2008). For a good overview of nanotechnology, see Pickrell (2006), and of how to use nanotechnology in the classroom, see Mallmann (2009).
This is all a long way from drilling holes in ceramics though, and when asked what he was going to do next, Professor Jerby replied: “I hope to generate energy from common materials in an efficient and practically feasible manner.” In the mean time, remember that any attempt to dry your nanotech socks using a microwave oven could lead to fireworks!
For some classroom experiments using microwave ovens, including the production of plasma balls, see Stanley (2009) in this issue.
The author would like to thank Professor Jerby, Tel Aviv University, Israel; Dr Narayanan from ESRF; and Dr Schrempp, from Los Osos High School, Rancho Cucamongo, California, USA, for help with this article.
issue12_nature_abrahamson2000.pdf
Download this article as a PDF