Supporting materials
Download
Download this article as a PDF

Tea is a refreshing drink – and it can also help students to learn about important chemical reactions, as these simple experiments with infusions demonstrate.
Teas, including black, herbal, and fruit teas, are popular drinks and come in a wide array of varieties, matched by an impressive variety of colours once brewed. As many people will have noticed, adding substances to tea can change its colour – and a colour change can indicate a chemical change.
In this article, we take a closer look at some chemical reactions – acid–base reactions, reduction/oxidation, and complex formation – that can be investigated by using simple and safe experiments with teas.

Tea is made by infusion: a physical method of extraction in which chemical compounds, such as oils and volatile organic compounds, dissolve in the infusing liquid. In the case of tea bags, the plant matter forming the material for infusion cannot pass through the bag, but smaller particles and compounds that cause colour and flavour can do so. Thus, the tea bag acts as a partially permeable membrane.
Traditionally, the term ‘tea’ referred to a product made by harvesting leaves from the tea plant (Camellia sinensis) that were then further processed. This product is now often referred to as ‘black tea’, because of its appearance before infusion. (This is distinct from tea served without milk, which confusingly is sometimes also known as black tea.) Today, however, many different plant-derived materials are marketed as teas, so in this article we use the term ‘tea’ to include all these products, and the term ‘black tea’ to refer specifically to traditional tea.
We devised the following tea-based activities for students aged 14–19. They are also suitable for ages 11–14 if there is less emphasis on the theory behind the chemical reactions. The activities can be completed in one lesson (approximately 60 minutes), and we recommend that students work in groups of 3–4. Optionally, students can use the video feature on their cell phones to record and review the changes they see.
The infusions should be prepared at the start of the lesson and allowed to cool down to room temperature before the activities are carried out. The activities can be introduced while the infusions are cooling. The following infusions should be prepared:
In this activity, we explore the colour changes that occur when an acid and a base are added to a striking blue-coloured infusion of butterfly pea flower tea.
For remote teaching, this activity could be done by older students at home. In this case, small glasses or white cups can be used instead of test tubes, and sodium hydrogencarbonate should be used instead of ammonium hydroxide because it is safer to handle, although the colour change is less striking.
To prepare this activity, each group requires:
Students should work through the following steps as a group:
Discuss the following questions with your students:
In the activity, students should see the following colour changes (see figure 1):
These colour changes occur because butterfly pea flower tea contains molecules called anthocyanins (see text box). These molecules alter the wavelength of light they absorb, and therefore their colour, depending on the pH of the solution they are in. Lemon juice contains citric and other acids, so adding this to the tea produces a more acidic (lower pH) solution and a visible colour change. Adding a weak base such as ammonium hydroxide or sodium hydrogencarbonate decreases the number of hydrogen ions, leading to a lower acidity (higher pH value) and another colour change.

Anthocyanins are a group of water-soluble pigments responsible for the red, blue, or purple colouration of many flowers, fruits (including blueberries and raspberries), and vegetables (such as red cabbage).[1] These pigments are also partly responsible for autumn leaf colours.
The region of these pigment molecules that produces the colour is called the chromophore. In the chromophore region, the energy difference between two molecular orbitals falls in the range of the visible-light spectrum. Adding a hydrogen ion to a chromophore can change its wavelength of absorbance, and thus its colour. Because the acidity of a solution (or pH value) depends on the concentration of hydrogen ions, chromophores – and the plant material containing them – potentially act as natural acid–base indicators.
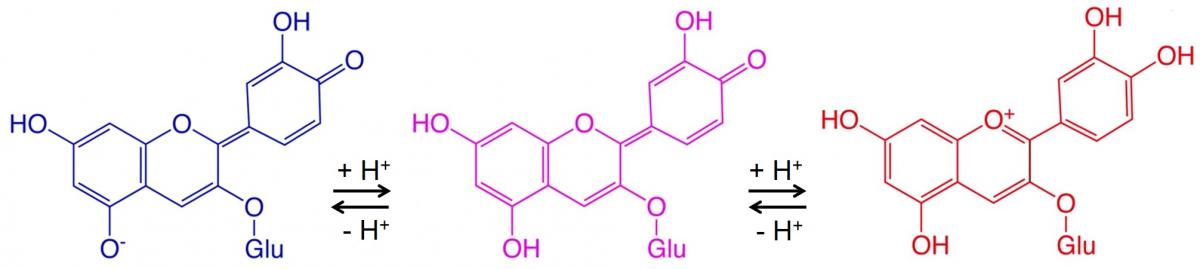
There are many examples of colour changes involving pH-sensitive anthocyanins and other vegetable dyes. Figure 2 shows an example.
Here, two reversible reactions, each involving adding a hydrogen ion to the molecule, cause a colour change at each step. Acidic conditions (low pH) drive the reversible reactions to the right (blue to red), while basic conditions (high pH) drive the reversible reactions to the left (red to blue).
This experiment uses a solution containing iron(III) ions to produce a colour change indicating the presence of polyphenols such as tannins in tea. The solution should be prepared by the teacher before the class by dissolving a teaspoon of iron(III) chloride hexahydrate in 50 ml of water. Alternatively, a steel pan scourer or steel wool can be used, although in these cases the colour change will be slower. When using the iron(III) solution, it is instantaneous.
Each group of students will need the following materials:
Students should work through the following steps:
Discuss the following questions with your students to explore the key concepts:
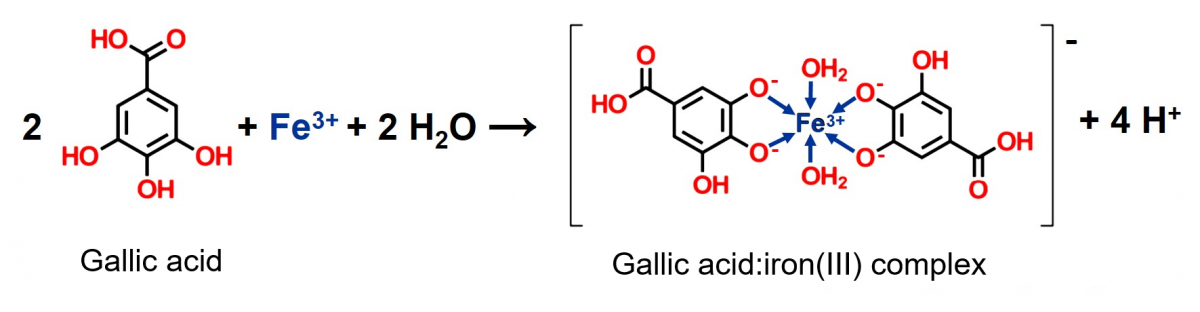
In the activity, the tea darkens when iron(III) ions are present, changing from pale brown to black, with the appearance of ink (see figure 3). The compounds responsible for this change are polyphenols, which react with the iron ions. Tea contains a number of polyphenols, including tannins that give it bitterness and astringency, and an example of these polyphenols is gallic acid and its derivatives, which are part of the structure of several tannins. The chemistry involved is rather complex, but in short, the gallic acid reacts with iron(III) ions to form ferric pyrogallate, a black insoluble complex ion.

This activity can be used as a starting point to discuss how transition metal complexes are formed (the type of bonding involved, what ligands are, and how pH changes affect formation).
Figure 4 shows an example of a complex of gallic acid and iron(III), with two molecules of water acting as co-ligands to complete the octahedral coordination sphere.[2]
You can also test other infusions for the presence of polyphenols, for example, those based on rooibos. Different colours, from dark brown to dark blue, may result depending on the iron(III) complexes formed.
In a previous article for Science in School ,[3] we introduced the idea of colour changes due to oxidizing and reducing agents (in that case caused by chemical reactions between glucose lollipops and permanganate salts).
In this activity, we look at colour changes due to the action of oxidizing and reducing agents on hibiscus tea. Here, sodium percarbonate acts as the oxidizing agent and sodium dithionite as the reducing agent.
Each group of students will need the following materials:
Students should work through the following steps:
Discuss with your students the following questions:
Students can also be asked to do their own research to investigate how this activity relates to bleaching products. For example:
The hibiscus tea discolours (bleaches) almost completely with sodium dithionite, but not with sodium percarbonate (figure 5).

Chemical bleaches are products used to remove colour from fabric and to clean stains. They react with many coloured organic compounds, including natural pigments. Oxidizing agents are most commonly used, but some reducing agents are also used.
Sodium percarbonate is a typical peroxide-based oxidizing bleach. The peroxide group gives rise to very reactive oxygen species, and these are the active bleaching (and oxidizing) agents. They break apart chemical bonds in the chromophore region of the pigment molecules (see text box), changing their colour.
A reducing bleach, such as sodium dithionite, works by converting the carbon–carbon double bonds in the chromophore to single bonds, thereby decreasing the oxidation state of the carbon.
Although most of the reagents are common household chemicals, some, such as ammonium hydroxide solution, sodium percarbonate, and sodium dithionite, can be irritants or corrosive. Teachers should follow their local health and safety rules and the advice on the product label. A lab coat, gloves, and safety glasses should be worn by all students to avoid contact of the chemicals with skin and eyes. Particular care should be taken with the iron(III) chloride. See also the general safety note on the Science in School website. All waste is safe for disposal down the drain.
This article is based on a presentation at the Spanish Science on Stage festival (Ciencia en Acción) in 2020 by Marisa Prolongo, helped by her nephew Guillermo and her niece Ana. The authors are grateful to the Technical University of Madrid (UPM) for support throughout the project ‘Promoting inquiry-based STEM learning’; to the ‘la Caixa’ Foundation for support with the project ‘Science and technology within everyone’s reach’; and to the Spanish Royal Societies of Physics (RSEF) and Chemistry (RSEQ).
[1] Rusishvili M et al. (2019) Unraveling the molecular mechanisms of color expression in anthocyanins, Physical Chemistry Chemical Physics 21: 8757-8766. doi: 10.1039/C9CP00747D
[2] Rattanakit P, Maungchang R (2019) Determining Iron(III) Concentration in a Green Chemistry Experiment Using Phyllanthus emblica (Indian Gooseberry) Extract and Spectrophotometry, Journal of Chemical Education 96:756-760. doi: 10.1021/acs.jchemed.8b00817
[3] Prolongo M, Pinto G (2018) Colourful chemistry: redox reactions with lollipops. Science in School 43: 41-45.
At Wellington College we have personally used the Teach Article ‘Colourful Chemistry: Redox Reactions with Lollipops’ which is written by the same authors. We have found that students really enjoyed the visual aspect and learning more about the chemistry behind the demo. As a result, I am excited to attempt the experiments in this new Teach article involving various teas. Our students are currently being taught remotely in the UK: Activity 1 can be carried out by students at home before the lesson and then provide the students chance to share their results.
Caroline Evans, Head of Chemistry, Wellington College, UK
Download this article as a PDF