How effective is your sunscreen? Teach article
Encourage students to stay safe in the sun with a collection of activities to discover the science behind sunscreen.
Whether it’s enjoying a summer’s day on the beach, skiing down the slopes in winter, or simply going outside for some fresh air during a lunch break, any time spent outdoors leaves our skin vulnerable to the Sun’s ultraviolet (UV) rays. Applying sunscreen is key to protecting ourselves from the damaging effects of sunlight, but have you ever stopped to consider how your sunscreen works – and ultimately, how effective it is?
To find out, we devised the following activities for students aged 14–17 to explore the science behind sunscreen and to investigate its effectiveness for UV protection. In the process, students will appreciate the importance of protecting themselves from sunlight, and will be more able to make informed decisions regarding their use of sunscreens. We recommend that students work in small groups (e.g. 2–3 students per group) throughout.
Activity 1: An introduction to UV protection
This activity introduces students to the key ingredients in sunscreens, and explores the difference between organic and inorganic UV filters. In small groups, students study the labels of various store-bought sunscreens to determine the key ingredients and types of UV filters that are commonly used. We recommend placing the sunscreen bottles around the classroom, with each group working their way around the different sunscreen products. You could collect your own selection of sunscreen products, or ask your students to bring in products from home. This activity (including a class discussion) takes approximately 50 minutes, and it is helpful to complete it before the main activities.
Materials
To have a diverse collection of sunscreens, try and find the following:
- One sunscreen with low protection (SPF 15 or below)
- One sunscreen with medium–high protection (SPF 30–50)
- One sunscreen with very high protection (SPF 50+)
- One sunscreen formulated for babies/children
- One sunscreen for water sports (high water-resistance)
- One sunscreen with titanium dioxide or zinc oxide (inorganic active ingredients)
Procedure
Ask your students to work through the following steps:
- In your group, begin by examining the label of the first sunscreen. Consider the following questions, and record your observations in a table (see table 1):
- What are the key pieces of information that are included on the front of the packaging?
- Does the sunscreen provide UVA protection, UVB protection, or both (broad spectrum)?
- What are the SPF number (e.g. 10, 15, 30) and SPF category (e.g. low, medium, high)?
- Looking at the ingredients list, what active ingredients are found in the sunscreen?w1
- What are the first 5 inactive ingredients listed on the label?
- Repeat the previous step for the different sunscreens around the classroom, and compare your findings.
- In your group, discuss the different characteristics of the sunscreens and try to classify them, taking into account the type of UV filter (UVA, UVB or broad spectrum), their active ingredients, and the SPF. You will share your preliminary conclusions with the rest of the class in a follow-up discussion.
| Station | Sunscreen type/brand | SPF value | SPF category | UVA/UVB/broad spectrum filters | Active ingredients | Inactive ingredients |
|---|---|---|---|---|---|---|
| 1 | Kids mineral sunscreen | 50 | High protection | Broad spectrum | Titanium oxide | Water, Glycerin, Propylene Glycol, Diisopropyl Sebacate, Ethylhexyl Salicylate |
| 2 | High-protection gel sunscreen | 50+ | Very high protection | UVA/UVB | Avobenzone, Octocrylene | Water, Alcohol Denat., Ethyhexyl Salicilate, Dibutyl Adipate, 4-Methylbenzylidene Camphor |
| 3 | Hydrate and protect face sunscreen | 15 | Medium protection | UVA/UVB | Avobenzone, Octinoxate, Oxybenzone | Water, Cyclopentasiloxane, Glycerin, Butyloctyl Salicylate, Dimethycone |
Discussion
Discuss the following questions with your students to explore the key concepts:
- Which substances are commonly used as UV filters (the active ingredients)?
- Are the active ingredients organic or inorganic substances?
- What are the main inactive ingredients found in sunscreens?
- How many of the sunscreens claimed UVA protection, UVB protection or both?
- What is the difference between UVA and UVB? What are their known health effects?
- What does ‘SPF’ mean? How is SPF calculated?
- What is the difference between a sunscreen with SPF 30 and a sunscreen with SPF 50?
Explanation
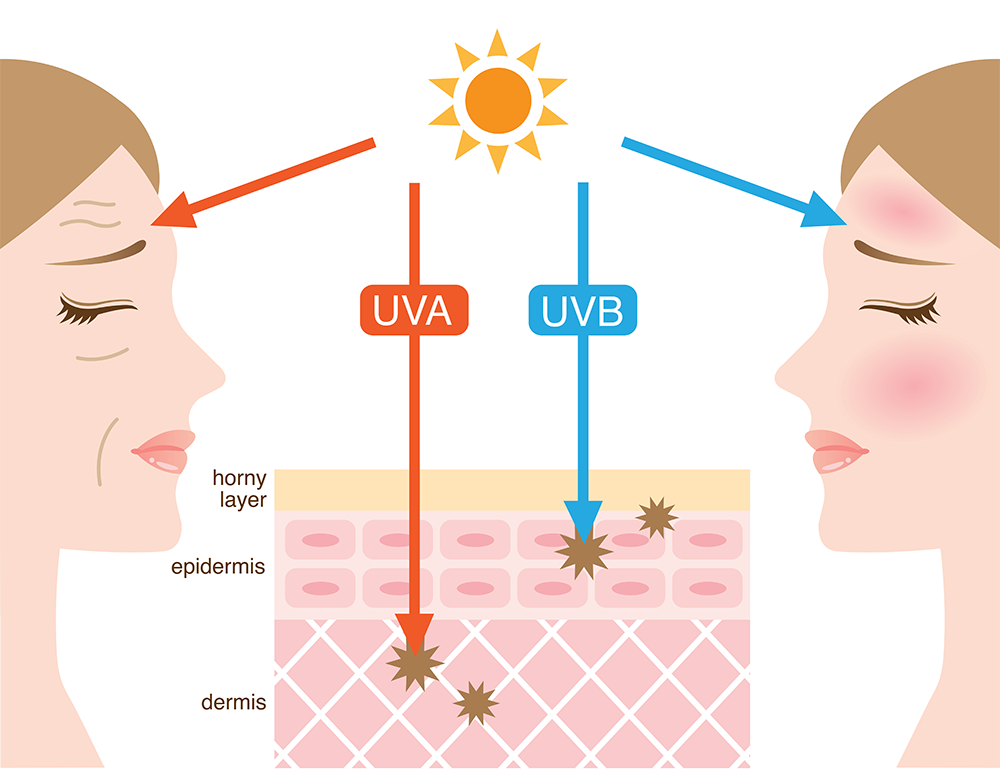
layer the dermis) resulting in skin ageing, while
UVB rays damage the upper layers of the skin
(the epidermis) causing sunburn.
yomogi1/Shutterstock.com
Sunscreens protect us from the Sun’s harmful rays by blocking or absorbing UV radiation. There are two types of UV radiation that can damage our skin: UVA and UVB. They affect our skin in different ways (UVA, for example, is the dominant ‘tanning’ ray, while UVB causes sunburn), and both types increase the risk of skin cancer. UVA rays are also responsible for premature ageing effects such as wrinkling.
At sea level, UVA comprises approximately 95% of the UV energy reaching Earth’s surface, and UVB comprises the remaining 5%. UVB rays have a wavelength of 280–320 nanometres (nm), while UVA rays are split into two categories: UVA1 (320–340 nm) and UVA2 (340–400 nm). SPF (sun protection factor) indicates a sunscreen’s ability to protect skin from UVB damagew2. It is a measure of how much radiation is required to cause sunburn on protected skin (i.e. with sunscreen) relative to the amount of radiation required to produce sunburn on unprotected skin. The higher the SPF, the more UVB protection the sunscreen provides. In addition to the SPF, sunscreens are now categorised as providing low to very high protection, to ensure the labels are easy to understand.
Since the SPF only measures protection against UVB rays, it is important to choose a sunscreen that also has high UVA protection. According to EU recommendations, the UVA protection for a sunscreen should be equivalent to at least one-third of the labelled SPF. Products that achieve this requirement are labelled with the letters ‘UVA’ printed in a circle. Sunscreens that protect from both UVA and UVB do so by either combining UVA and UVB filters or by using a broad-spectrum filter.
In addition to being classified according to their SPF and UVA protection, sunscreens can be grouped depending on whether their active ingredients are organic or inorganic. Organic UV filters are a group of carbon-containing compounds that absorb UV radiation and convert it to heat energy. Inorganic filters, on the other hand, are a group of mineral oxides such as zinc oxide and titanium dioxide, which reflect UV radiation.
The active ingredients are combined with a ‘base cream’, which is made up of various inactive ingredients. This forms a product that can be easily applied to skin.
Activity 2: Formulating sunscreen
In this activity, students formulate their own inorganic sunscreen at two SPF values using zinc oxide. The aim is to learn more about the composition of sunscreens, while also exploring key concepts such as concentration, solubility, polarity and emulsions. This activity will take approximately 50 minutes.
Materials
To prepare one sample of sunscreen, each group requires:
- Lanette wax (contains a mixture of cetearyl alcohol and cetostearyl alcohol)
- Sweet almond oil
- Liquid paraffin
- Glycerine
- Distilled water
- Zinc oxide
- Water bath heated to 80 ºC
- Two 250 ml beakers
- Three 50 ml beakers
- Two glass rods
- Magnetic stirrer
- Spatula
- Electronic balance
Procedure
Ask your students to work through the following steps:
- Begin by preparing an emulsion that will serve as the base cream of your sunscreen. Add the oily phase ingredients to a 250 ml beaker: 15 g lanette wax, 7 g sweet almond oil and 7 g liquid paraffin. Weigh out the ingredients using an electronic balance.
- Place the beaker inside the water bath. Mix the ingredients using a glass rod or magnetic stirrer for 5 minutes until the mixture is well combined. Leave the beaker inside the water bath.
- In a separate 250 ml beaker, add the aqueous phase ingredients: 5 g glycerine and 66 ml distilled water. Mix using a new glass rod or the magnetic stirrer.
- Keeping the oily-phase beaker in the water bath, pour the aqueous-phase solution into the oily phase, stirring continuously for 5–10 minutes until you have a homogeneous mixture: this is the base cream. Leave the beaker inside the water bath.
- In a 50 ml beaker, mix the zinc oxide with liquid paraffin in a ratio of 5:4, i.e. 2 g of zinc oxide to 2 ml (1.6 g) of paraffin.
- In a separate 50 ml beaker, measure out 4.1 g of the base cream. Add 0.9 g of zinc oxide/paraffin paste and mix them with a glass rod until combined. This creates a sunscreen that is 10% zinc oxide, which equates to an SPF of approximately 10.
- In another 50 ml beaker, measure out 3.2 g of base cream and add 1.8 g of zinc oxide/paraffin paste. Mix them with a glass rod until combined. This creates a sunscreen that is 20% zinc oxide, which equates to an SPF of approximately 20. You will use these sunscreens in the next activity.

placed in a beaker inside a
water bath and mixed using a
magnetic stirrer or glass rod.
Fina Guitart

solution with the oily phase
solution produces the
homogenous base cream.
Fina Guitart

produce their own zinc oxide
sunscreen to test the
effectiveness of sunscreen.
Fina Guitart
Discussion
Discuss the following questions with your students to explore the key concepts:
- Which base cream ingredients are soluble only in water? Which are soluble only in oil? Justify your answers in relation to the polarity of the substance.
- Why is lanette wax essential for your formulation?
- What are the characteristics of emulsions and emulsifiers?
- What is the difference between an oil-in-water and a water-in-oil emulsion?
- Find two emulsifiers in the ingredient lists of some sunscreens from activity 1.
Explanation
The base cream produced in the activity is an emulsion, since it has an oily phase and an aqueous phase. The emulsifier (which stabilises the emulsion) is lanette wax. Emulsifiers typically have a polar (or hydrophilic) head and a non-polar (or hydrophobic) tail, and they tend to have greater or less solubility in either water or oil. The lanette wax emulsifier is more soluble in oil, although also water-soluble. Emulsifiers that are more soluble in oil tend to form water-in-oil emulsions, whereas emulsifiers that are more soluble in water tend to form oil-in-water emulsions.
Activity 3: Investigating the effectiveness of UV protection
The aim of this activity is to investigate the effectiveness of the two sunscreens produced in activity 2. Using colour-changing craft beads that change colour in response to UV light (at 300–360 nm) to represent our skin, students can determine how well the two sunscreens shield the beads from UV radiation, and thus how well they protect. We found it was best to use purple or blue beads as they show changes in colour intensity best. Use the same colour beads for all experiments, i.e. all blue or all purple. Note that all UV beads start off white before they are exposed to light. The procedure takes approximately 50 minutes.
Once students have tested their own sunscreens, they should expand their investigations (see extension section) to compare a variety of other store-bought sunscreens.
Materials
Each group requires:
- Sunscreens from activity 2
- 10–12 UV beads (kept in a light-impenetrable container)
- Small transparent plastic sheets (large enough to cover four UV beads)
- Petri dish or similar (to hold the UV beads)
- Colour chartw3 or smartphone with a colour detection app (such as ‘Color Grab’ or ‘Drop – Colour Palette’)
- UV light source, e.g. torch or black light
- Spatula
- Laboratory stand and clamps
- Stopwatch
Safety note
Do not look directly towards UV lamps. Turn on the UV lamps only when required, and after safety instructions have been given by teachers.
Procedure
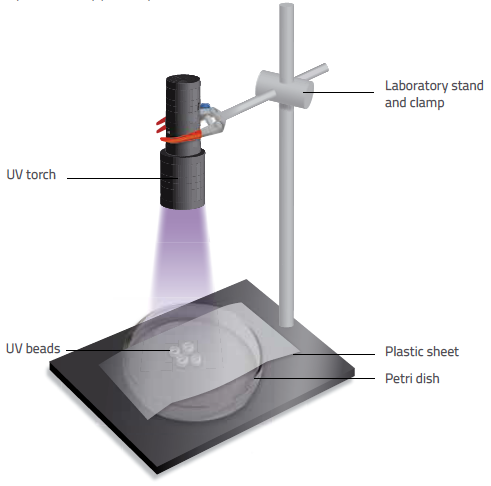
Nicola Graf/Fina Guitart
Ask your students to work through the following steps:
- Place four UV beads in the centre of a petri dish. Position the UV lamp approximately 20 cm away from the dish, or clamp a UV torch to a stand positioned at the same distance. Ensure that the UV light source stays at the same distance throughout the experiment and that the light always illuminates downwards.
- Collect a pea-sized amount of your first sunscreen from activity 2 (10% zinc oxide) using a spatula, and spread a thin layer of the sunscreen onto the centre of the plastic sheet to cover the UV beads (approximately 4 cm in diameter).
- Place the plastic sheet on top of the beads, ensuring that the sunscreen covers the beads.
- Turn on the UV light source for 5 seconds, keeping count with a stopwatch.
- As soon as the time is up, turn off the lamp and use the colour chart or a colour detection app to record the colour of each bead. Alternatively, take a photo of the beads to compare their colours with those obtained in subsequent experiments. Ensure that you save your data and/or photos.
- Move the UV beads into a dark container so that they are not exposed to light. Add four new, unexposed beads to the petri dish.
- Repeat the procedure twice more for your first sunscreen so that you conduct the experiment three times in total.
- Follow the same procedure for your second sunscreen (20% zinc oxide), and also for two controls: one using a clean plastic sheet without sunscreen, and another using no plastic sheet.
- Compare the colour intensities of the UV beads for each sunscreen and the controls.

Fina Guitart
Discussion
Discuss the following questions with your students:
- What conclusions can you draw from your experiments?
- Are your experimental results consistent with the idea of SPF?
- What were the dependent, independent and fixed variables in your experiments?
- Why should UV beads be used only as a guide to compare the effectiveness of sunscreens? What are the main disadvantages of this experimental method?
Explanation
The experiment should show a clear difference in the colour of the beads in the presence and absence of the homemade sunscreens, and students should be able to observe a small difference between the use of sunscreens of 10% zinc oxide and 20% zinc oxide. The darker the colour of the beads, the more UV radiation they have absorbed, and the less effective the sunscreen. This shows students that there is a difference in the amount of UV radiation that can be absorbed by the beads, depending on the SPF of the sunscreen. This was more evident when we tested store-bought sunscreens of the same brand and type (but different SPF) in our extension experiments.
We found there was no significant difference in outcomes between the two control experiments, which tested the UV beads with and without the plastic sheet. However, using a UV sensor (see extension section), students may detect a slight difference in the amount of UV radiation reaching the beads with and without the plastic sheet.
Extension activity
Students can devise their own investigations and create new hypotheses by expanding on the previous procedure to test other sunscreens. For example:
- Compare two store-bought sunscreens (choose among sunscreens of activity 1), one of SPF 15 and the other of SPF 30, keeping the brand and sunscreen type the same.
- Compare two sunscreens of the same SPF but from two different brands.
- Compare two sunscreens of the same SPF, brand and type, but with a difference in ‘shelf life’, e.g. using one sunscreen that has only just been opened, and one that has expired.
You can make further modifications to the experiments, for example:
- Test the UV beads in sunlight, rather than under a UV lamp or torch.
- Using the same procedure, test various pairs of sunglasses to investigate their effects against UV light.
- For a more sophisticated method, use a UV sensor to investigate the effectiveness of sunscreens by measuring the intensity of UV light. Place the plastic sunscreen sheet over the sensor, rather than the UV beads, and record the sensor’s readings.
Web References
- w1 – For a list of active ingredients used in sunscreens, visit the US Food and Drug Administration (FDA) website to download a poster that outlines their proposed changes to sunscreen regulations.
- w2 – Learn more about SPF and the factors related to sun exposure on the FDA website.
- w3 – For activity 3, students can use a colour chart such as this one created by the Royal Society of Chemistry. Note that students should ignore the ‘UV Sun index’ scale.
Resources
- Visit the British Association of Dermatologists website for a factsheet on sunscreen, including details of how to apply sunscreen and tips for staying safe in the sun.
Review
This article is a great example of a cross-curricular project that offers learning in an active way. The activities provide the perfect mix of discussion, analysis and experiment on the topic of sunscreens and their UV protection – something that is highly relevant to teenagers.
The experiments and discussions can be used to explore a variety of topics that are central to most science curricula, and they also provide the opportunity to learn about the scientific method. The time needed for all three activities is approximately 3 hours, which – from my point of view as a teacher of chemistry, physics and biology – is time well spent.
Dr Ingela Bursjöö, science teacher, Montessori school Elyseum, Gothenburg, Sweden





