Ancient signal-sensing mechanisms based on cyclic dinucleotide molecules may lead to breakthroughs in human healthcare Understand article
From ancient bacteria to humans, cyclic dinucleotide second messenger signalling molecules are key to lifestyle regulation and disease. This makes them an attractive target for new medicines.
Human quality of life has probably never been better. However, despite the eradication of some of the most devastating infections through hygiene and vaccination measures, not all infectious diseases can be eliminated. A number of viruses, such as HIV and the viruses that cause influenza, the common cold, and herpes, efficiently infect a large percentage of the human population. While some of these viruses may not cause severe disease in healthy people, they can be severe in those with a weakened immune system. Many other diseases, including cancer, autoimmune diseases, and allergies also affect the quality of life of millions around the world. Lastly, lifestyle-associated diseases, such as obesity, diabetes, and cardiovascular disease, are a major cause of human suffering by themselves and also increase susceptibility to infections, including biofilm-associated chronic microbial infections (figure 1).
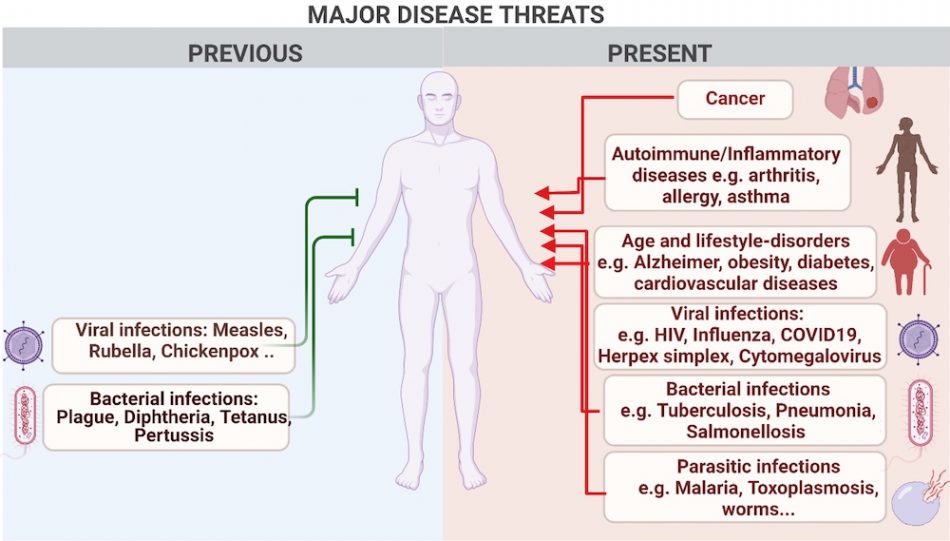
Image courtesy of the authors, produced with Biorender
Common signalling mechanisms
Do cancer, autoimmune diseases, viral infections, and biofilm infections have a common factor? While not obvious at first sight, recent molecular investigations have revealed the existence of common sensing and signalling mechanisms that, when dysregulated, can cause disease.
The processing and storage of information did not start with the computer era. All lifeforms, from bacteria to humans, depend on some form of signal transduction. Surprisingly, some of the principles of signal transduction are conserved from bacteria to higher organisms, including humans.[1-4]
At least nine different senses are known in humans, including the classical five senses: auditory, olfactory, gustatory, visual, and tactile cognition (figure 2).
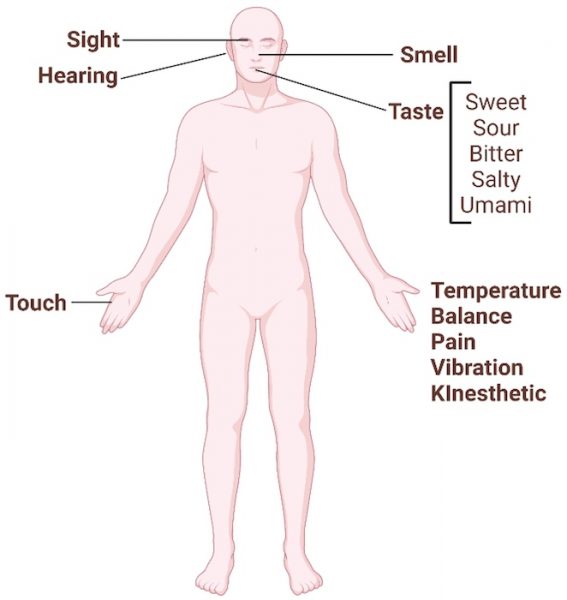
Image courtesy of the authors, produced with Biorender
An initial signal, for example, from a glucose molecule that causes a sweet taste on the tongue or light of a certain wavelength that we see as red, requires a receptor to sense and recognize it. This signal perception causes a molecular signal cascade inside cells and results in the enzymatic synthesis of second messengers. These are small diffusible molecules that mediate distinct physiological responses (figure 3A), and one such family of molecules is the cyclic dinucleotides (CDNs).[4-6]
Ribonucleotides
The genetic information to build our body and life is written in DNA, which consists of a sequence built from four deoxyribonucleotides. Transcription of this information gives short-lived complementary RNA sequences built from the four corresponding ribonucleotides: adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP).
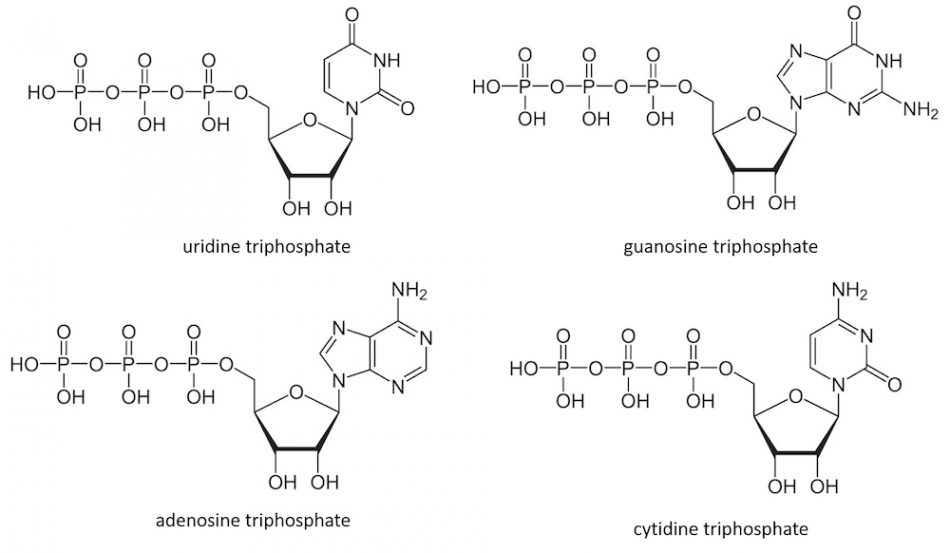
However, these molecules have important functions beyond our genetics; for example, ATP is also the major energy currency of cells. Nucleoside triphosphates are the building blocks of cyclic nucleotides (CDNs), which consist of combinations of two nucleoside monophosphates connected by two phosphodiester bonds between the ribose units. These CDNs are key signalling molecules in all branches of life.
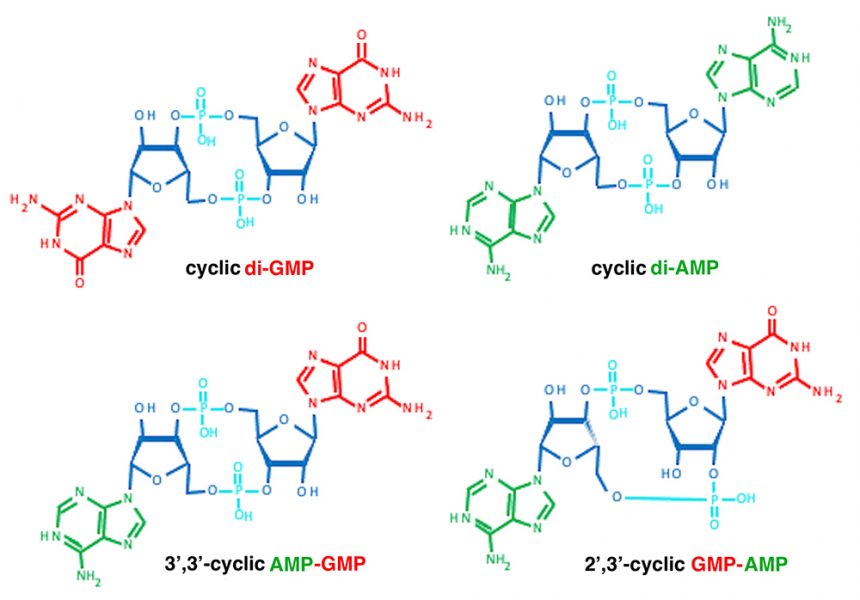
Image: Structures obtained from https://pubchem.ncbi.nlm.nih.gov
DNA sensing in health and disease
Cells infected with either a bacterium or a DNA virus show similar signalling mechanisms. Our own DNA is normally only found in the nucleus and mitochondria; viral or bacterial DNA in the cytoplasm is ‘out-of-place’ and is sensed by the cGAS enzyme. In response, cGAS synthesizes a CDN second messenger (2’,3’-cGMP-AMP; figure 3A and B). This second messenger in turn binds to the central sensory protein STING, which leads to the activation of antiviral and antitumor immunity.[4-6]
In addition to infections, out-of-place DNA in the cytoplasm can occur under other cell-stress situations, such as exposure to DNA-damaging conditions like radiation, excessive sunlight, and chemotherapy. DNA stress also occurs during chromosome duplications, in cancerous cells, and during the natural process of cell aging. Furthermore, genetic mutations in the key enzymes that continuously clean out-of-place DNA from the cell can result in the accumulation of DNA in the cytoplasm, which triggers chronic inflammation. This, may contribute to the development of autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, or age-associated neuronal disorders, such as Alzheimer’s disease (figure 3B).
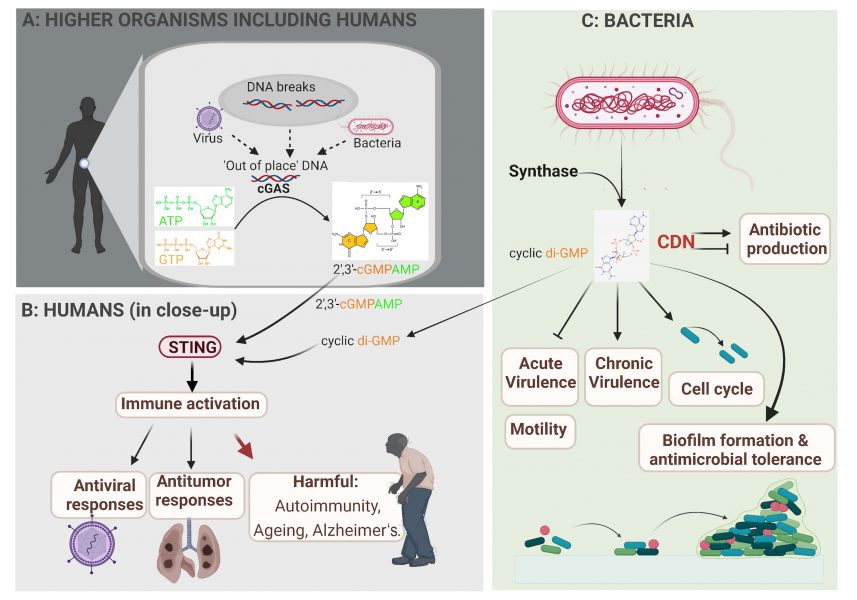
Image courtesy of the authors, produced with Biorender
Bacteria sense in a similar way
Bacteria also have highly sophisticated signalling cascades for signal perception and conversion[1, 2, 7] in which CDN second messenger molecules play key roles (figure 3C). For example, the bacterial CDN molecule cyclic di-GMP (along with other, less frequently occurring CDNs) is so common in bacteria that it acts as a microbe-associated molecular pattern molecule that helps alert our immune system to bacterial infection. Cyclic di-GMP directs the fundamental microbial lifestyle transition between motility and sessility at the single-cell level. Eventually, this transition leads to multicellular biofilms that make infections by these bacteria harder to treat. The transition towards biofilm formation is accompanied by alterations in many physiological processes, including the regulation of photosynthesis in cyanobacteria; changes in antibiotic production; the regulation of virulence factors in human, animal, and plant pathogens; and phage infectivity in commensal bacteria (figure 3C).
From sensing to healing
The key role of CDN second messengers in signal transduction provides an opportunity to develop treatment strategies. For example, the cyclic di-GMP pathway in bacterial pathogens can be disrupted to eradicate biofilm infections. Moreover, in humans, CDN-mediated responses that lead to autoimmunity and accelerated aging could be tackled by using inhibitor compounds. Activation of the CDN second messenger system is currently under clinical trial as a potential treatment against viral infections and cancer.
The functionality of CDNs as second messengers is widespread and ancient. These heat-resistant molecules were present in thermophilic microbes known to be some of the earliest lifeforms on Earth. Knowing more about them could give us insights into where we come from and how we can cure ourselves.
References
[1] Kresge N, Simoni RD, Hill RL (2005) Earl W. Southerland’s discovery of cyclic adenine monophosphate and the second messenger system. J Biol Chem. 280: E39–E40. doi: 10.1016/S0021-9258(19)48258-6
[2] Ross P et al. (1987) Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325: 279–281. doi: 10.1038/325279a0
[3] Römling U, Galperin MY, Gomelsky M (2013) Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 77: 1–52. doi: 10.1128/mmbr.00043-12
[4] Ablasser A et al. (2013) cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498: 380–384. doi: 10.1038/nature12306
[5] Gekara NO, Jiang H (2019) The innate immune DNA sensor cGAS: A membrane, cytosolic, or nuclear protein? Sci Signal. 12: eaax3521. doi: 10.1126/scisignal.aax3521
[6] Danilchanka O, Mekalanos JJ (2013) Cyclic dinucleotides and the innate immune response. Cell. 154: 962–970. doi: 10.1016/j.cell.2013.08.014
[7] Witte G et al. (2008) Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell. 30: 167–178. doi: 10.1016/j.molcel.2008.02.020
Resources
- Watch a video introduction to cell signalling as background to this article.
- Learn about the discovery of the first cyclic nucleotide second messenger molecule was awarded with a Nobel Prize in 1971.
- Read more Understand articles about biomedical research:
- Schmerbeck S et al. (2021) Organ-on-chip systems and the 3Rs. Science in School 54.
- Le Guillou I (2021) Clinical trials count on more than statistics. Science in School 52.
- Chugh P (2019) Cells: why shape matters. Science in School 46: 8–13.
- Amponsah PS (2016) Cellular redox – living chemistry. Science in School 36: 15–17.





