Colour in nature: think pink Understand article
What do carrots and flamingos have in common? At first glance, not much, but look closer. Their rosy glows have surprisingly similar origins!
Have you ever wondered where the colours in nature come from? Why are some plants green and others red, or why some animals are black, white, or spotted?
In nature, each colour exists for a reason, depending on the species, on environmental conditions, and on evolution.
These colours all have something in common, however: the science behind them!
Each colour we perceive arises from a small region of the electromagnetic spectrum called the visible region. The wavelengths in this region, from about 400 to 700 nm, are associated with various colours. Collectively, they appear as “white light”.
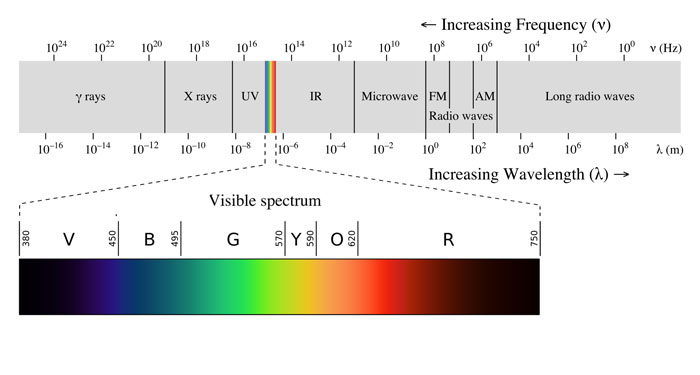
Image: Philip Ronan, Gringer/Wikimedia, CC BY-SA 3.0
When a coloured object absorbs visible light, it reflects certain wavelengths. This reflected portion reaches the retina in our eyes and corresponds to the colour we see. The retina has two photoreceptors, rods and cones, that allow us to perceive different colours and luminosities.
So, what about pink in nature? In plants, flowers, and animals, this colour is strictly connected to chemistry and to several classes of molecules that can induce different shades of pink.
Pink chemistry: Anthocyanins, betalains, and haemoglobins
The first class of molecules are anthocyanins, water-soluble dyes belonging to the flavonoid family and present in some plants and flowers.[1] Anthocyanins are responsible for several colours ranging from orange-pink-red (pelargonidin), through mauve (cyanidin), to dark purple-blue (delphinidin).
Some anthocyanins are only found in certain flowers, such as the peonidin found in some pink Chinese peony cultivars. Most anthocyanins have the ability to change colour depending on the pH. For this reason, some flowers containing this pigment can change colour from blue to pink depending on the soil pH.
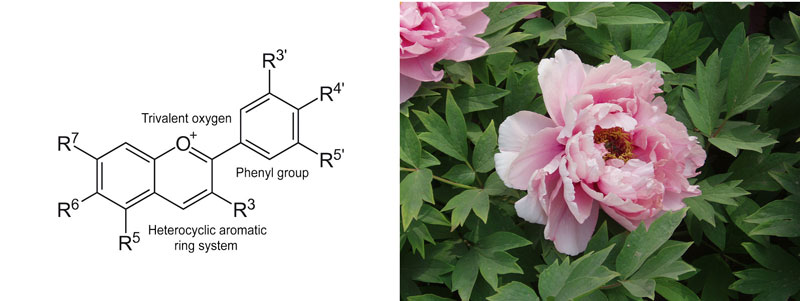
Anthocyanin structure: modified from NEUROtiker/Wikimedia, Public Domain. Peony: Fanghong/Wikimedia, CC BY-SA 3.0
An example of this behaviour is seen in hydrangeas, a large group of flowering plants that originated in Asia and the Americas. Hydrangea flowers are usually white, but may display a variety of pink, red, purple, and blue hues owing to interactions between pink anthocyanins in the plant and aluminium ions in the soil. At low pH (below 7), the plant can absorb aluminium ions and the flowers become blue due to the formation of a complex between anthocyanins and aluminium. In basic soil (with a pH above 7), however, the aluminium ions are less available to the plant so the flowers appear pink or red. This behaviour earned them the nickname ‘change rose’. [2]

Image: Matthias Böckel/Pixabay
Another class of molecules responsible for pink hues in flowers is betalains, a group of powerful antioxidants divided into two categories: betacyanin, with colours ranging from reddish to purple, and betaxantine, with colours ranging from yellow to orange.
This pigment can be found in many plants of the order Caryophyllales, such as bougainvillaea and some succulents (including cacti),[3] and are also present in large quantities in beetroot. The most abundant betacyanin in beetroot is bethanin, which is also used as a natural dye with the name “beetroot red”.

Betalains and anthocyanins are found only in plants, and have the same role: they attract pollinating insects with bright colours and protect the flowers from ultraviolet rays by absorbing them. However, anthocyanins and betalains have never been found simultaneously in any plant.[3]
Many animals also have pink hues on their skin or plumage, so what’s behind their colours? In animals, colour may arise from molecules such as haemoglobins, proteins present in the red blood cells of all vertebrate animals and in the circulatory fluids of many invertebrates (worms, some arthropods, echinoderms, and some molluscs). They are responsible for the pink to red colours seen in the combs and wattles of birds and the skin of some primates.
Haemoglobins have the ability to combine with atmospheric oxygen in the lungs, gills, or other respiratory surfaces of the body and to deliver oxygen to tissues. Each haemoglobin molecule contains four iron ions (Fe2+) that can transport oxygen from the lungs to the tissues and carbon dioxide from the tissues to the lungs. Due to the presence of iron, haemoglobin has a pinkish-red colour. The intensity of the colour increases when the iron is bound to oxygen and decreases when it is bound to carbon dioxide.
What links flamingos and carrots?
There is a class of natural pigments that connects the plant world to the animal world: carotenoids! This is a class of more than 750 naturally occurring pigments synthesized by plants, algae, and photosynthetic bacteria. They have a common structure composed of a long chain of conjugated double bonds with carbon ring structures at one or both ends of the molecule.[4] This structure is crucial for light absorption and is the reason why carotenoids are colourful.
When a molecule absorbs light energy, its electrons, which normally reside in an energetic level called the “ground state”, are pushed to higher energy levels and the molecule enters an “excited state”. The more highly conjugated the system, the lower the energy difference between these two states and, consequently, a lower energy of light needed is needed to excite the electrons. In molecules with many conjugated multiple bonds, this light absorption may occur in the visible region; the remaining light reaches our eyes and we perceive a colour.
Small differences in the chemical structure of carotenoids can shift their colour from yellow to orange, pink, and red. Carotenoids are present in carrots, of course, but also in tomatoes, peaches, and grapefruits!
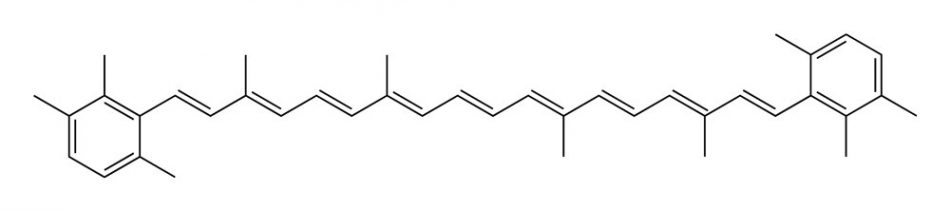
Image: Ben Mills/Wikimedia, Public domain
Interestingly, one carotenoid is found in both plants and animals: astaxanthin. It is synthesized by a microalgae, Haematococcus pluvialis, which produces it to neutralize the free radicals that form during exposure to UV rays in sunlight and protect the algae from damage. Astaxanthin is a microalgae sun cream!
So how does this carotenoid pass from plants to animals? Through their diet. This microalgae is the favourite food of animals such as crustaceans, which are often pink in colour due to their astaxanthin-rich diet.
One such very tiny crustacean, Artemisia salina, is the favourite food of two fascinating animals: Phoenicopterus roseus, better known as the flamingo, and Platalea ajaja, also called the roseate spoonbill. These birds are born white and become pink as they start to eat A. salina (and the microalgae, too). Astaxanthin from the A. salina is absorbed in fats deposited in the birds’ feathers, bills, and legs, and this causes their singular pink tint.

Image: Kyaw Tun/Unsplash

Image: Lip Kee/ Flickr CC BY-SA 2.0
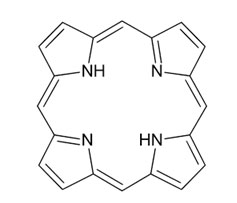
The pink colour in most birds’ plumage is caused by carotenoids, but there are some exceptions, such as Tauraco erythrolophus (the red-crested turaco), with its intensely pink-coloured head. This bright colour is due to the presence of turacin, the copper salt of a porphyrin secreted by the turacos in their wing feathers.
Porphyrins all share a common structural unit: a heterocyclic skeleton consisting of four pyrrole molecules joined by methine bridges. Porphyrins are responsible for a range of colours, including pink, brown, red, and green, and are found in many birds, such as owls, pigeons, and gallinaceous species.[5]
Defence, mimicry, and mistakes: the dark side of pink
The colour pink may be exploited in nature for several reasons, for example, as a form of defence. This is the case in Desmoxytes purpurosea, also known as the dragon millipede, which is a spiny, toxic millipede with shining pink legs. But watch out! This colour advertises a deadly weapon: cyanide. The millipede produces hydrogen cyanide from its defensive glands to protect it from predators, which also the reason for its almond smell (a typical aroma of cyanides).
Another animal that uses pink for defensive purposesis Dactylopius coccus, also called the cochineal insect, which lives on cactus plants in Peru and the Canary Islands. The bright colour comes from carminic acid that the insect produces as a chemical weapon against predation. This backfired for the insect though, since they are traditionally used to produce the red dye cochineal.

Image: Cleveland museum of Art, Public Domain
Conversely, some species use pink to blend in, such as Hymenopus coronatus, the Malaysian Orchid Mantis. This insect is not only a praying mantis, but is one of the most sophisticated examples of mimicry found in nature. After the first moult, it turns from red-orange to translucent white with soft pink shades. Its legs develop petal-like expansions, a series of brownish longitudinal streaks appear on the upper part of the abdomen and the insect becomes in all respects similar to an orchid flower. In this way it obtains a double advantage: as a predator, it can surprise bees, flies, and butterflies; as prey, it hides from birds, mammals, and reptiles that hunt by sight.[7] Another master of camouflage is Hippocampus bargibanti, a smaller species of seahorse only about two centimetres in length. A large number of pink-coloured tubercles on its bright white body makes it resemble in all respects the corals on which it lives.
Sometimes nature can make mistakes, however, as with Amblycorypha oblongifolia, a pink grasshopper. There is only a one in 500 chance of encountering this insect. It is not a rare species or a newly discovered animal; its colour is due to a genetic mutation called “erythrism” which causes excessive production of red pigments to take over the green colour. However, this is very disadvantageous to the grasshopper. Being so gaudy, it struggles to blend in among the leaves and becomes easy prey.

Image: Frupus/Flickr CC BY-NC 2.0

Image: Richard Whitby/Flickr CC BY-SA 2.0
In short, whether it is wanted or by mistake, nature can give you any shade of pink you want!
References
[1] de Pascual-Teresa S, Sanchez-Ballesta MT (2008) Anthocyanins: from plant to health. Phytochem. Rev. 7: 281–299. doi: 10.1665/034.022.0106.
[2] Chenery EM (1948) Aluminium in Plants and its Relation to Plant Pigments. Annals of Botany 12: 121–136. doi: 10.1093/oxfordjournals.aob.a083177.
[3] Burchi G, Ballarin A Trinchello D (2006) Flower colour: factors that control its expression and techniques that can modify it. Italus Hortus 13: 3–17.
[4] Cazzonelli C (2011) Carotenoids in nature: insights from plants and beyond. Funct Plant Biol. 38: 833–847. doi: 10.1071/FP11192.
[5] Tahoun M et al. (2021) Chemistry of porphyrins in fossil plants and animals. RSC Adv. 11: 7552–7563. doi: 10.1039/D0RA10688G.
[6] Srisonchai R et al (2018) A revision of dragon millipedes I: genus Desmoxytes Chamberlin, 1923, with the description of eight new species (Diplopoda, Polydesmida, Paradoxosomatidae). ZooKeys 761: 1–177. doi: 10.3897/zookeys.761.24214.
[7] O’hanlon JC, Norma-Rashid Y (2013) Coloration and Morphology of the Orchid Mantis Hymenopus coronatus (Mantodea: Hymenopodidae). Journal of Orthoptera Research 22: 35–44. doi: 10.1665/034.022.0106
Resources
- Read a Teach article on indigo history and how you can extract it at school: Farusi G (2012) Indigo: recreating Pharaoh’s dye. Science in School 24: 40–46.
- Use thin-layer chromatography in your class and discover the pigments that give leaves their colour: Tarragó-Celada J, Fernández Novell JM (2019) Colour, chlorophyll and chromatography. Science in School 47: 41–45.
- Make pH-sensitive inks from fruits and vegetables thanks to anthocyanins: Giraldi Shimamoto G, Vitorino Rossi A (2015) An artistic introduction to anthocyanin inks. Science in School 31: 32–36.
- Find experiment ideas for your Earth Science class: King C, Devon E, Kennett P (2010) Getting down to Earth: ideas for the earth science classroom. Science in School 15: 39–43.
- Read an article on the colour of flamingos from Science Focus.
- 125 things that are pink in nature.
- An article on Scientific American about the oblong-winged katydids and how pink seems to be the dominant colour in the North American species.
- A SciShow video on natural things that are intensely coloured because of their structure and not for the presence of pigments.
- Watch a video reflecting on why mammals are not as colourful as other species.