Purple fumes: the importance of iodine Understand article
Iodine, with its characteristic purple vapours, has myriad applications – from the familiar disinfectant to innovative solar cells.

Image courtesy of Eleanor A
Merritt
What makes iodine so important and interesting? Not only does it sublimate into a dramatic purple gas, but it also affects many aspects of life on Earth and of human civilisation. Did you know, for example, that iodine protects marine algae from oxidative damage (for example from the Sun), prevents some congenital abnormalities in humans, and has many industrial applications?
The discovery of iodine can be traced back to the 19th century and the Napoleonic wars. With the British imposing a blockade on European ports, the French were faced with shortages of saltpetre (KNO3) for manufacturing gunpowder. So chemist Bernard Courtois investigated the potential of seaweed (brown algae, Laminaria sp.) as the potassium source for this crucial substance. He added concentrated sulfuric acid to seaweed ash and was surprised by the beautiful purple fumes that were produced.

Lussac, French physicist and
chemist, by François Séraphin
Delpech (1778–1825)
Public domain image /
Wikimedia Commons
Although Courtois suspected that his purple vapour was a new element, he did not have the financial means to follow up his research. It was left to his colleagues, including Joseph Gay-Lussac, to confirm his results and name the element iodine, from the Greek word iodes, which means purple or violet.
Gay-Lussac went on to investigate the chemistry of iodine, and despite the war, the French chemists found ways to correspond with British chemists, notably Sir Humphry Davy. Initially, Davy believed the vapour to be a chlorine compound, but soon concluded that it was indeed a new element.

the strongest iodine
accumulators among living
systems. Photograph taken
on the shore at Dunstaffnage,
near Oban, Scotland, UK
Image courtesy of FCK
With the help of X-ray absorption spectroscopy, we now know that seaweeds accumulate iodine as iodide (I-), which acts as an antioxidant to protect them against oxidative damage caused by atmospheric ozone (O3). This goes some way to explaining why trace amounts of molecular iodine (I2) can be detected in the atmosphere of coastal regions and why human iodine intake in these regions is dependent on seaweed abundance rather than proximity to the sea.
For much of the next century, iodine continued to be extracted from seaweed. Today, however, it is removed from natural iodine-containing brines in gas and oil fields in Japan and the USA, or from Chilean caliches (nitrate ores), which contain calcium iodate (Ca(IO3)2). The iodine is supplied to the market as a purplish-black solid.
Iodine chemistry
Iodine belongs to the halogens, and thus shares many of the typical characteristics of the elements in this group. Because of its high electronegativity, iodine forms iodides with most elements in its formal oxidation state, -1. Many iodine-containing compounds are frequently used as reagents in organic synthesis – mainly for iodination, oxidation and C–C bond formation.
Iodine in the atmosphere originates mostly from biological and chemical processes in the ocean – such as the iodide antioxidant system in seaweeds. Most iodine is ultimately removed from the atmosphere by cloud formation. In the ocean, iodine is mainly dissolved and exists as iodate (IO3–, oxidised form) and iodide (I–, reduced form). In Earth’s outer layer (the lithosphere), most iodine is in marine and terrestrial sediments; iodine levels are low in igneous rocks.
The physiological importance of iodine
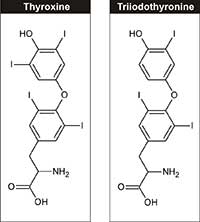
thyroxine (T4) and
triiodotyronine (T3)
Image courtesy of Michael
Zimmermann
Physiologically, iodine is an essential element, required for the synthesis of thyroid hormones – triiodotyronine and thyroxine – which regulate growth, development and cell metabolism. The recommended dietary intake of iodine for adults is 150 µg/day, which can be obtained from dairy products, seaweed and iodised table salt.
The classic symptom of iodine deficiency is thyroid enlargement (goitre). As iodine intake falls, the anterior pituitary gland secretes increasing levels of thyroid-stimulating hormone in an effort to maximise the uptake of available iodine; this leads to excessive growth of the thyroid gland.
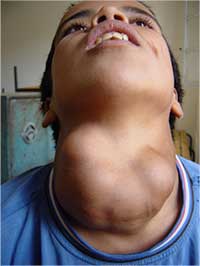
goitre caused by iodine
deficiency
Image courtesy of Michael
Zimmermann
But the most damaging effect of a lack of iodine is to the developing brains of babies, leading to mental retardation. Furthermore, severe iodine deficiency during pregnancy is associated with a greater incidence of stillbirth, miscarriage and congenital abnormalities.
The most effective way to prevent iodine deficiency is to add potassium iodide (KI) or potassium iodate (KIO3) to table salt. This practice of salt iodisation is carried out in around 120 countries, with more than 70% of the world population now having access to iodised salt.
Industrial uses of iodine
Iodine and its compounds are used in myriad products, from food and pharmaceuticals, through to animal feed and industrial catalysts. For instance, iodine is a potent antimicrobial. For more than a century, iodine tincture – a mixture of ethanol, water, iodine and potassium iodide – was used as an antiseptic for wounds. This has now largely been replaced by water-soluble ionophores (iodine complexed with surfactants), which are less irritating to the skin. For example, povidone iodine, a mixture of polyvinylpyrrolidone and iodine, is used widely as a surgical scrub.
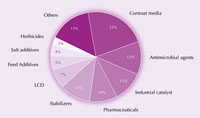
iodine. Click on image to
enlarge.
Image courtesy of Tatsuo Kaiho
In the industrial production of acetic acid, iodine compounds such as rhodium iodide (the Monsanto process) or iridium iodide (BP’s Cativa process) are used to catalyse the carbonylation of methanol.
Silver iodide (AgI), used in early photographic plates, is used today in cloud seeding to initiate rain and to control climate. Because AgI has a similar crystal structure to ice, it can induce freezing by providing nucleation sites. This was done at the 2008 Beijing Olympics to prevent rainfall during the opening and closing ceremonies.

two silver iodide generators
on the sides for cloud
seeding.
Image courtesy of Christian
Jansky/ Wikimedia
With its high atomic weight (126.9) and large number of electrons, iodine is also an excellent X-ray absorber and is used in X-ray contrast media. These substances are generally safe to administer to humans and enable the visualisation of soft tissues in X-ray examinations.
A more everyday application of iodine is in liquid-crystal displays for TVs, computers and mobile phones, which use polarising films to filter light. These films are commonly made of polyvinyl alcohol layers doped with iodine. Here, iodine acts as a cross-linker and ensures that the structure is polarising.
Iodine in the energy industry

Tricastin, France, is situated
close to a densely populated
region. Approximately every
five years, potassium iodide
pills are distributed to the
people who live nearby to
prevent damage to their
thyroid glands in case of a
nuclear accident.
Image courtesy of the AIEA/
Wikimedia
Iodine is used in one of the most promising solar cells on the market for the production of low-cost ‘green energy’: the dye-sensitised titanium oxide solar cell. Also known as the Grätzel cell after one of its inventors, it consists of polyiodide electrolytes as the charge transport layer between the cathode and the anode (to learn more, see Shallcross et al., 2009).
Of the 37 known isotopes of iodine, all but one, 127I, are radioactive. Most of these radioisotopes, which are produced via fission reactions in nuclear power plants and weapons, are short-lived, which makes them useful as tracers and therapeutic agents in medicine. For example, iodine isotopes can be used to image the thyroid gland, which absorbs radioactive iodine when it is injected into the bloodstream.
Unfortunately, radioactive 131I, released from nuclear accidents – such as the disaster in Fukushima, Japan, in 2011 – is also taken up by the thyroid. Because it is a high-energy β-particle emitter, it damages cells and induces cancer. To counteract this effect, non-radioactive potassium iodide (KI) tablets are ingested to saturate the thyroid’s ability to take up radioactive iodine.
These are just a small sample of the many applications of iodine. Clearly, although the element has been known for only two hundred years, it is well established in modern chemistry, physics and medicine.
Iodine in the classroom
No doubt we are all familiar with the colourful ‘iodine clock’ experiment between hydrogen peroxide, potassium iodide, starch and sodium thiosulphate – but there are many other ways to introduce iodine practically into the classroom. For example:
- When catalysed by water, aluminium and iodine react to produce spectacular clouds of purple iodine vapour.
- In a direct reaction between a metal and a non-metal, zinc powder reacts with a solution of iodine in ethanol to form zinc iodide in an exothermic redox reaction.
- Potassium iodide can be used to detect the presence of starch in a range of foods.
- Various solutions, including aqueous sodium iodide, can be electrolysed and the products at the electrodes identified. Students can then use their practical experience and theoretical knowledge to construct simple ionic equations.
Details of these and many other school experiments can be downloaded from the Learn Chemistry websitew1.
Acknowledgement
This article was adapted from a much longer publication in Angewandte Chemie International Edition (Küpper et al., 2011).
References
- Küpper FC, Feiters MC, Olofsson B, Kaiho T, Yanagida S, Zimmermann MB, Carpenter LJ, Luther GW, Lu Z, Jonsson M, Kloo L (2011) Commemorating two centuries of iodine research: an interdisciplinary overview of current research. Angewandte Chemie International Edition 50: 11598-11620. doi: 10.1002/anie.201100028
- Shallcross D, Harrison T, Henshaw S, Sellou L (2009) Looking to the heavens: climate change experiments. Science in School 12: 34-39.
Web References
- w1 – The Learn Chemistry website of the UK’s Royal Society of Chemistry offers a wide range of downloadable resources to support the teaching and learning of chemistry.
Review
In this concise update on the element iodine, the authors guide the reader through the history and the many applications of this important element, from medicine to industry and energy production. Suggestions for school laboratory experiments add interest and appeal to the topic.
Given the plain and clear style, I recommend this article not only to European science teachers but also to their students aged 13-18. It could be used in lessons on chemistry (the periodic table, halogens), biology (endocrine glands, the thyroid and its diseases) and physics (isotopes, radioactivity and solar cells). There is also an interdisciplinary opportunity to address the history of science (the discovery of the elements), the role of scientists in the development of weapons, or the relationships between scientists of opposing countries during wartime.
Suitable comprehension questions include:
- From the article you can deduce that seaweeds accumulate iodine:
- To oxidise atmospheric ozone
- To absorb atmospheric ozone
- To produce atmospheric ozone
- To protect themselves from atmospheric ozone
- If we do not receive enough iodine:
- Our thyroid gland enlarges / atrophies
- Our anterior pituitary gland secretes less / more thyroid-stimulating hormone
Giulia Realdon, Italy





