Supporting materials
Download
Download this article as a PDF

Oscillating reactions: an unusual and fascinating topic to explore.
“We had shown to Primo Levi a clock reaction. He had never seen one. There are chemical reactions in which you mix substances in a beaker, and an orange colour comes out, for example. Then you wait a few seconds and the colour disappears; after a few more seconds it reappears, then disappears to reappear again, and so on. They are called clock reactions; they are characterised by very precise timescales, and are incredible from a chemical point of view, because chemical reactions normally do not exhibit these characteristics. Primo Levi, curious as he was, was very amazed. […] They are periodic reactions. Normally in chemistry you start from the reactants and end up with the products. Not in this case: you have an oscillating phenomenon, and it is astonishing. Some clock reactions have truly remarkable theoretical importance. Primo Levi came to us to see them.”
From an interview with Professor Adriano Zecchina, University of Turin.[1]
Primo Levi was one of the greatest Italian writers of the twentieth century. His most famous books are precious testimonies of the horrors of World War II. However, his major literary output is intimately connected to the scientific field, given that Levi was a chemist. His book The Periodic Table was awarded as best science book of all times by the Royal Institution of Great Britain.[2]
Chemical clocks, also known as clock reactions, are a class of chemical reactions following nonlinear dynamics. They are characterised by periodic (recurring) variations in the concentrations of some chemical species involved, typically intermediates and/or catalysts. They are a typical example of complex phenomena in chemistry and are of considerable biological importance: they are involved in many rhythmic-electrical phenomena (e.g., impulses and heartbeats) and are the basis of the striped or spotted coats of many animals (e.g., stripes on a zebra’s coat or spots on a leopard’s fur). Therefore, understanding them could clarify many of the dynamics of the living world. The history of the discovery of chemical oscillators is also very interesting. For more detailed background information on this topic, refer to info sheet 1.
Figure 1 shows an example of a typical catalysed chemical reaction with intermediate chemical species. The concentration of the reagents (A and B) and the intermediate chemical species (I1, I2, etc.) decreases continuously over time (downward-pointing arrows), while the concentration of the products (C and D) increases continuously over time (upward-pointing arrows). The catalyst acts as both a reactant and product, and the circular arrows indicate that it reforms at each reaction cycle.
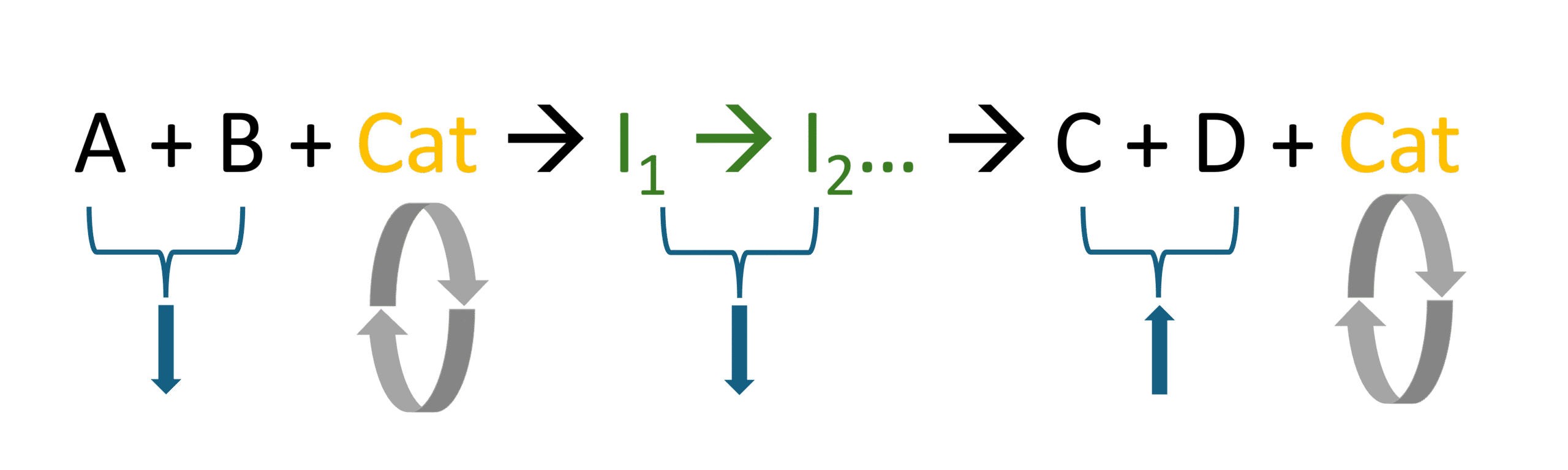
Figure 2 shows an example of a catalysed chemical clock with intermediate chemical species. The downward-pointing arrows indicate a continuous decrease in reactants, while upward-pointing arrows indicate a continuous increase in products. The wavy lines represent the periodic oscillations of concentrations of the catalyst and/or intermediates. The concentration of the intermediates decreases non-linearly over time. The catalyst is regenerated at the end of a reaction cycle, but its concentration can also change in an unusual way. The concentrations of the catalyst and/or the intermediates have minimums and maximums. The amplitude of the oscillations decreases over time. You can find a simple explanation of the dynamics of oscillating reactions in info sheet 2.
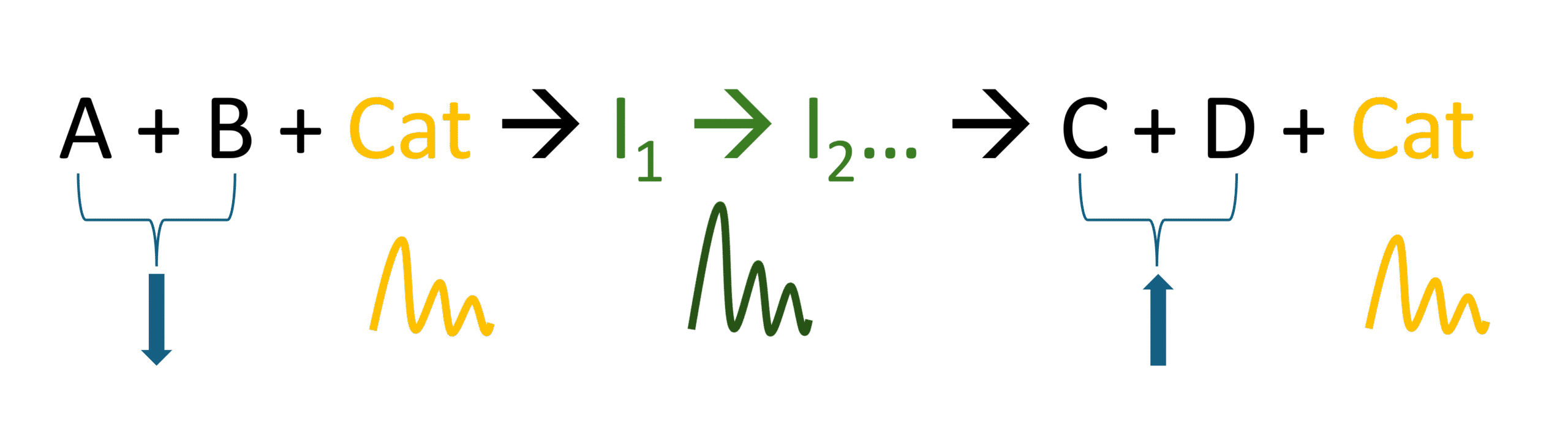
It’s easy to observe periodic variations in the concentration of the catalyst and intermediates when these chemical species are coloured, as in the famous Belousov-Zhabotinsky (BZ) reaction.[3] Since the BZ reaction requires reagents not always available in schools, the following example illustrates the procedure for a chemical clock that can be performed with readily available reagents[4] (figure 3). The colour changes resulting from the oscillations in concentration are not very evident, but they can be observed with a little attention. In contrast, the moment at which the oscillations end is very clear, as the solution suddenly turns very dark.

Although the experiment is simple to perform, the chemistry required for even a general understanding of the reaction mechanism is quite complex. For this reason, this activity is intended for upper secondary students (16–19 years old) who have already dealt with chemical kinetics and chemical equilibrium.
The reported example fits the explanation of chemical kinetics and can also be used when discussing redox chemistry, the descriptive chemistry of iodine and the chemistry of vitamin C, paying particular attention to its biological role. The biological implications of chemical clocks make this topic truly interdisciplinary.
This activity should take around 15 minutes.

Wear a lab coat, safety glasses and gloves.
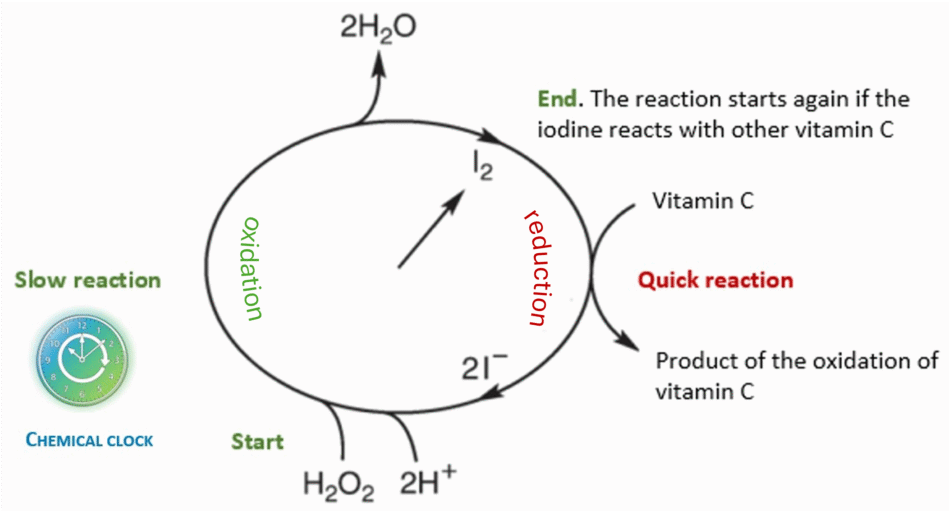
While performing the experiment, you may observe some colour changes. In Step 2, the red colour of iodine disappears instantly in the presence of vitamin C (ascorbic acid). This is a redox reaction in which iodine is reduced and vitamin C is oxidised.
This quick reaction between vitamin C and iodine is:
(A)
Then, the oxidation product of ascorbic acid rapidly hydrolyses:
(B)
In Step 4, a slow reaction occurs: the iodide ion (produced by the dissociation of the hydroiodic acid obtained in reaction A) catalyses the decomposition of hydrogen peroxide into water and oxygen (H2O2 (aq) → H2O(l) + O2(g)), generating both iodide ions and elemental iodine (see info sheet 3). The iodide ion and elemental iodine generate the triiodide ion (reaction C). The triiodide ion is in the so-called ‘tincture of iodine’: elemental iodine is apolar and therefore not soluble in polar solvents except in the presence of iodide. For simplification, the triiodide ion is often referred to as elemental iodine I2.
(C)
I2 + I– → I3–
Although reaction C is usually represented with a single arrow, it is actually an equilibrium reaction.
The presence of the triiodide ion is revealed by the appearance of a characteristic blue-black colour due to the formation of a complex with starch, as presented in (D).
(D)
2I3– + starch (light) → starch–I5– (blue-black) + I–
Before observing the characteristic blue-black colour due to the stable formation of the triiodide ion, the colour of some streaks in the mixture varies, oscillating between a light and a darker shade (see Video 1).
Here is a possible explanation:[5] The slightly dark colour indicates that the triiodide ion is forming and beginning to bind to the starch. Due to the reaction path going back and forth several times, a pulsation of light and dark shades is created until the blue-black triiodide-starch complex forms permanently. Evidently, this is due to periodic oscillations in the concentration of certain chemical species according to autocatalytic and/or autoinhibitory stages that are still not fully understood (see info sheet 3 for some information about the most accredited reaction mechanism). Once the oscillations of the concentrations cease, the colour darkens definitively.
The graph of Figure 4 can be useful to have a unified picture of the reactions involved.
Below are some questions for students (Worksheet 1). Example answers can be found in the supporting material.
Chemical clocks are typically not taught in secondary schools because they are considered too difficult to understand. However, they can be presented simply and without oversimplification, allowing students to grasp the complexity of the topic without being intimidated by it. This hands-on activity enables students to perform an experiment that is both easy to reproduce and has an immediate impact. Students can carry out the experimental sequence more than once, making variations in the conditions. The historical background makes the topic particularly fascinating, demonstrating how the path of science is fraught with difficulties but also marked by fortunate circumstances. Finally, the biological implications of chemical clocks offer students a truly interdisciplinary and stimulating vision.
The activity was first proposed in the context of a career guidance initiative promoted by the Department of Chemistry of the Sapienza University (Rome), with the aim of encouraging enrolments on a chemistry Bachelor’s course (Call no. 16/2022 – Rep. no.126/2022 – Prot. no. 1662/VII/1, 22/06/2022 of Sapienza University). I was able to design and carry out some ad hoc activities, taking care of both the theoretical and practical parts. I am grateful for the opportunity to have actively participated in the program.
Furthermore, this case study was proposed during the internship activities at the Department of Science at Roma Tre University, where the experiment was carried out and discussed by several groups of trainee upper secondary school teachers (academic year 2024/2025).
[1] Interview with Professor Adriano Zecchina about Primo Levi and chemical oscillators: http://www.memoro.org/it/video.php?ID=2089
[2] Massi L, da Silva Lima G (2025) Science Communication As Praxis: Analysis of ‘The Periodic Table’ by Primo Levi. Substantia 9: 111-120. doi: 10.36253/Substantia-3441
[3] Video on the Belousov-Zhabotinsky reaction: https://www.youtube.com/watch?v=PpyKSRo8Iec
[4] Wright SW, Reedy P (2002) The vitamin C clock reaction. J. Chem. Educ. 79: 41-43. doi: 10.1021/ed079p41
[5] Ruekberg B (2020) A Closer Examination of the Mechanism of the Hydrogen Peroxide Iodine-Clock Reaction with Respect to the Role of Hypoiodite Species. J. Chem. Educ. 97:1688-1693. doi: 10.1021/acs.jchemed.9b00006
The article on chemical clock reactions and Primo Levi provides an excellent opportunity to develop ‘critical scientific literacy’ (CSL). Topics such as thermodynamics, reaction rate and catalysis can be explored, offering students an insight into the human side of science, as experienced by Levi. Simulating or observing these reactions helps students to engage actively and to think like scientists, which is an important skill for the future.
Giorgia Messori, ITIS E.Fermi, Italy
Download this article as a PDF

Use a lollipop to activate colour-changing redox reactions in this simple but eye-catching…
Discover simple adaptations to apparatus and experiments that make practical chemistry more accessible to students with vision impairment.

Iodine, with its characteristic purple vapours, has myriad applications – from the familiar disinfectant to innovative solar…