Faster, cheaper, CRISPR: the new gene technology revolution Understand article
A controversial new technology is making gene editing far cheaper and easier – too easy, perhaps?

You are typing a sentence and realise that a letter has been mistyped. The mistake will alter the sentence’s meaning, so you direct the cursor to the incorrect letter, press backspace and input the correct character. It is an exceedingly easy task. Amazingly, this simple approach is now being replicated in laboratories, with scientists in the role of authors and DNA as the message being modified.
This is the reality offered by CRISPR-Cas9, a new technology that has taken the scientific community by storm over the past few years. As well as promising important biomedical advances, it is now posing some difficult problems. Indeed, so much media coverage has already been generated by this controversial technology that science teachers may soon find themselves fielding questions from students about CRISPR-Cas9. So here we provide a quick guide to what CRISPR-Cas9 is and why it’s important.
What is CRISPR–Cas9?
CRISPR-Cas9 is a system that bacteria use to defend against viral attacks, but it has been exploited recently as a technology to snip genomes at precise points. The system comprises two components: CRISPR and Cas9. CRISPR stands for ‘clustered regularly interspaced short palindromic repeats’ and refers to locations on a genome where the DNA sequence repeats. Near these repeats are Cas genes, which code for important enzymes in the system. One of these, Cas9, is a nuclease – that is, an enzyme that cuts nucleic acid (DNA or RNA).
When a virus attacks a bacterium, it injects its nucleic acid into the bacterium, which responds by producing Cas enzymes to snip out pieces of the viral nucleic acid and incorporate them into its own genome at the CRISPR locations (figure 1). This provides some useful acquired immunity for the bacterium: the next time the same species of virus attacks, the CRISPR locations, along with the viral nucleic acid, are copied into short RNA molecules. These short RNA molecules first attach to the Cas9 enzyme and then guide the resulting complex to the invading nucleic acid, identifying it by its matching sequence. Cas9 then cuts the targeted nucleic acid, thus disabling the virus’s ability to hijack the bacterium for its own replication.
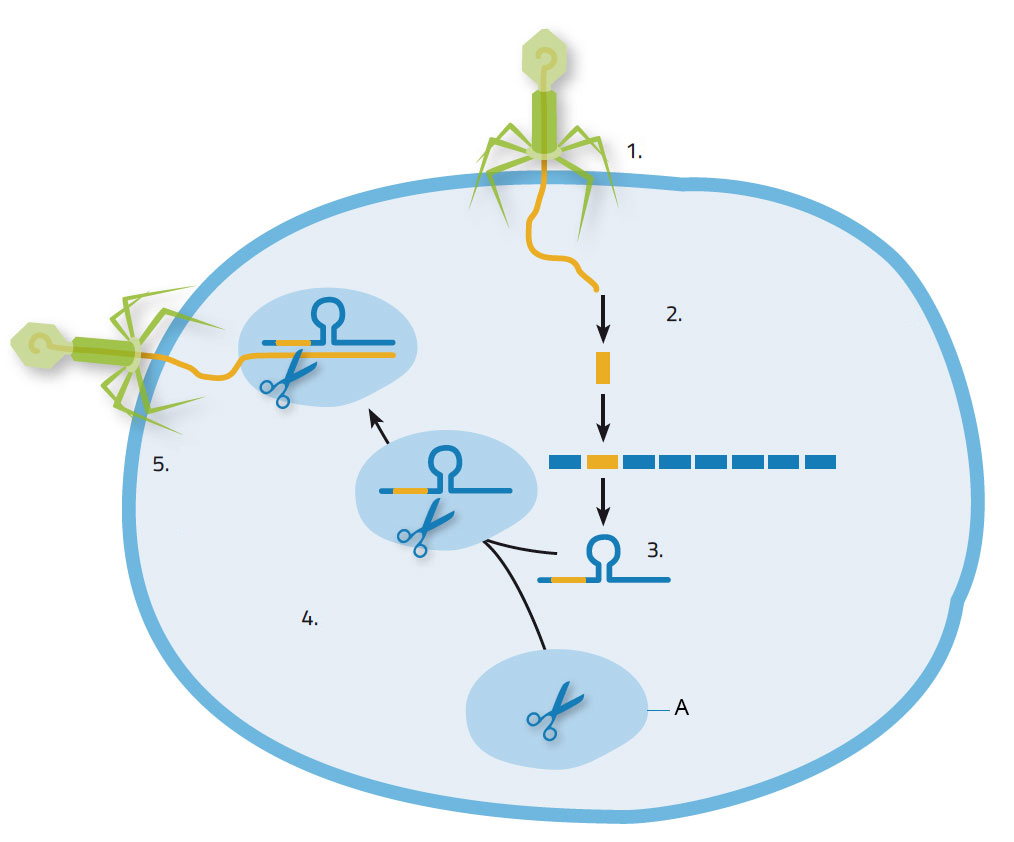
1. Virus invades bacterial cell; 2. Nucleic acid derived from virus is integrated into bacterial nucleic acid at CRISPR location; 3. CRISPR RNA is formed; 4.CRISPR RNA attaches to Cas9 enzyme; 5. CRISPR RNA guides the Cas9 enzyme to the virus. It cuts and destroys the viral genome.
Image courtesy of Nicola Graf
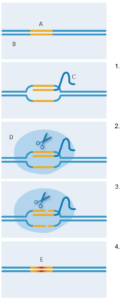
CRISPR-Cas9.
A: Target sequence; B: DNA;
C: Guide RNA; D: Cas9;
E: New DNA sequence
1. Guide RNA binds to target
DNA sequence
2. Cas9 enzyme binds to
guide RNA
3. Cas9 enzyme cuts both
strands of DNA
4. The bacterial repair system
introduces new DNA at the
strand breaks, replacing the
original DNA sequence.
Image courtesy of Nicola Graf
But how was this bacterial system adapted to provide a new genetic technology? In 2012, the teams of Jennifer Doudna at University of California Berkeley, USA, and Emmanuelle Charpentier, then at Sweden’s Umea University, modified and pieced together the short RNA molecules into a single ‘guide’ RNA (figure 2). This retained one end that could link to Cas9, but the sequences on the other end could be made to pair with any known target DNA. With this customising capacity, CRISPR-Cas9 could specifically cut DNA sequences of choice. Shortly afterwards, Feng Zhang’s lab at the Massachusetts Institute of Technology, USA, took CRISPR-Cas9 further by showing its capacity to induce precise genomic cuts in human and mouse cells (Cong et al, 2013). In addition, they tweaked Cas9 so that it would cut DNA in a slightly different way, stimulating a specific DNA repair mechanism in cells. This means the researchers successfully inserted a new DNA sequence precisely into the cut site, replacing the original sequence (figure 2).
With these pioneering efforts, CRISPR-Cas9 was transformed from being a niche topic in microbiology into an exciting research tool that enables researchers to edit genes for different purposes with great specificity and ease.
What is it used for?
To study genetic function, scientists often try to produce cell lines or model organisms in which the gene of interest is mutated or inactivated (a technique known as gene knockout). CRISPR-Cas9 provides a rapid and precise way of doing this. Additionally, the Cas9 enzyme can be modified so that it loses its DNA cutting ability but is still able to target and bind to specific DNA sequences, thanks to the guide RNA. This has the effect of physically blocking the binding of RNA polymerase, thus allowing scientists to control gene transcription – the starting point for gene activity.
While these are important techniques in biomedical research, CRISPR-Cas9 also promises more direct applications in health care. Scientists have already used CRISPR–Cas9 gene editing to excise HIV sequences and prevent the virus from replicating in human cell lines. It has also been used to remove mutated sequences in mice afflicted with Duchenne muscular dystrophy, which causes muscle weakness, thus presenting therapeutic hopes for patients and families suffering from this and similar genetic diseases. Recently, pig embryos have undergone extensive gene editing using CRISPR-Cas9 in the hope of providing safer organs for human transplantation.
So if editing has been done in pig embryos, could it also be done in human embryos? This is precisely what Chinese researchers did in 2015, when they used CRISPR-Cas9 to modify the β-thalassaemia gene in 86 human embryos (Cyranoski & Reardon, 2015). The technique turned out to be rather inefficient, as the DNA sequence was changed successfully in less than a quarter of the embryos. Nonetheless, the announcement of this finding generated much controversy because of the ethical implications.
Ethical issues – and the future
The ethical concerns relate not only to human embryo experimentation, but also to the uneasy prospect of germline engineering. With CRISPR-Cas9, it is theoretically possible to make genetic changes in germ cells (sperm and ova), which could be passed down to future generations. While this idea has great potential to eradicate genetic diseases, it also prompts the question of which modifications should be permitted as a medical treatment in the future. Could traits such as intelligence or eye colour be considered appropriate for medical improvement? These fears may seem far-fetched, but there is another worry: that germline engineering could have completely unanticipated and irreversible effects on future generations. This is particularly relevant since recent genetic research has found that interactions between genes and other hereditary mechanisms are far more complex than previously expected.
These implications have not escaped the attention of scientists. Some agree that germline engineering is fraught with ethical landmines and believe that any CRISPR-Cas9 research using human embryos should be halted. Others, however, believe that a complete moratorium would not only be difficult to enforce, but would also hinder research progress. Despite such differing viewpoints, the scientific community appears to have taken dialogue and transparency as the way forward, as evidenced by the many summits and meetings on this topic worldwide.
Meanwhile, the development of CRISPR-Cas9 continues surging forward. Pharmaceutical companies are investing in the technology to aid drug development research, and technical flaws are being addressed and improved – while a bitter patent dispute is playing out for the technique’s pioneers (Doudna/Charpentier and Zhang). Regardless of what happens there, the meteoric rise of CRISPR–Cas9 is only just beginning.
References
- Cong L et al. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–23. doi: 10.1126/science.123
- Cyranoski D, Reardon S (2015) Chinese scientists genetically modify human embryos. Nature 22 April, doi:10.1038/nature.2015.17378
Resources
- Read more on the potential use of CRISPR in RNA gene editing.
- Read more about the patent dispute regarding the CRISPR-Cas9 technology.
- Learn about how CRISPR-Cas9 technology is used to treat Duchenne muscular dystrophy.
- Read about how CRISPR-Cas9 is used in producing human transplant organs in pigs.
- Read an accessible article on the ethical issues of gene editing in germ cells.
- For an article featuring comments from international gene scientists on ethical issues of gene editing in germ cells, see:
- Bosley K et al. (2015) CRISPR germline engineering – the community speaks. Nature Biotechnology 33: 478–86
- Read more about Emmanuelle Charpentier’s revolutionary gene editing technique, and listen to her discussing her work.
Review
CRISPR-Cas9 is a powerful ‘GPS-like’ gene editing tool. It has the potential to open doors to many scientific, medical and agricultural applications. The relative simplicity and low cost of the technique raises important ethical issues. It has also resulted in a high-profile lawsuit between scientists over the valuable patent rights.
The article clearly describes the identification of palindromic repeats as a basic bacterial defence system for the application of the programmable CRISPR-Cas9 gene editing tool. The article can be used to explain, in conjunction with animations, the molecular biology techniques involved and why CRISPR-Cas9 has replaced earlier gene editing methods. It can also form the basis for discussions on ethical issues around germline editing and intellectual property. Comprehension questions around the article could include:
- What does CRISPR stand for?
- How does CRISPR-Cas9 work?
- What type of diseases can be treated using CRISPR-Cas9?
- What are the potential risks of using CRISPR-Cas9?
- What are the ethical issues surrounding the use of CRISPR-Cas9?
- Who should be awarded the Nobel Prize for the discovery of CRISPR-Cas9?
Mary Brenan, Concord College, UK





