Under pressure: the role of Earth’s mantle in our climate Understand article
Studies of iron oxides under extreme conditions are shining a light on Earth’s interior and its role in our climate.
Earth’s climate is affected by gases in the atmosphere, such as carbon dioxide, water vapour and methane. Less obvious, however, is the effect of rocks that lie deep below Earth’s surface. That’s not surprising: taking direct measurements of Earth’s interior is difficult, as is reproducing experimentally the conditions of high pressure and temperature that are found there. However, an international team of scientists has recently reconstructed the behaviour of the iron oxides inside Earth’s mantle using state-of-the-art equipment at the European Synchrotron Radiation Facility (ESRF)w1 in Grenoble, France (Bykova et al, 2016). With this information, the researchers are now beginning to understand the role of these chemical compounds in our climate.
Naturally occurring iron oxides are found in many different forms. “The most common iron oxide is hematite, Fe2O3, which is the end product of many geological processes and the main source of iron for our civilisation,” explains team member Elena Bykova from the University of Bayreuth, Germany. In recent years, scientists have discovered other iron oxides, such as Fe4O5, Fe5O6 and Fe13O19, that also form at high pressures and temperatures.
Previous geochemical studies have focused on hematite because, despite its simple chemical composition, it undergoes mysterious structural and electronic changes at high pressures and temperatures. Until recently, however, many investigations had failed to provide a consistent picture of the high-pressure behaviour of this material.
Elena and her colleagues used what is known as a diamond anvil cell (figure 1) to apply a pressure of more than 67 GPa to a hematite sample and heat it to more than 2400 °C. Using X-ray diffraction, the scientists showed that under these conditions, which correspond to those found 1500 km below Earth’s surface, hematite decomposes and forms Fe5O7, a previously unknown iron oxide, releasing oxygen in the process:
10 Fe2O3 → 4 Fe5O7 + O2↑
High-pressure experiments with another naturally occurring iron oxide, Fe3O4, showed that it also decomposes when heated at pressures above about 70 GPa (which represents conditions approximately 1670 km below the surface), forming Fe25O32 and releasing oxygen:
25 Fe3O4 → 3 Fe25O32 + 2 O2↑
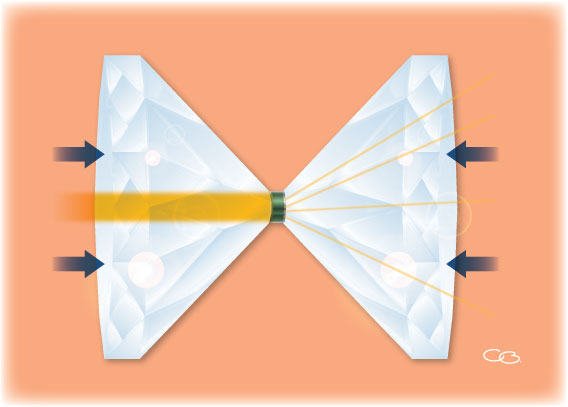
Image courtesy of ESRF / Format Editions
These results have important implications not only for fundamental high-pressure chemistry, but also for geology. One of our main sources of iron are huge sedimentary rock formations known as banded iron formations (BIFs), which consist of up to 50 % hematite as well as magnetite (Fe3O4). BIFs are found on all continents and can be several hundred metres thick and hundreds of kilometres long. Deposited on the ocean floor about two billion (109) years ago, they have since been forced further down by tectonic plate movement to depths of up to 2880 km below Earth’s surface, at the core-mantle boundary. The results of Elena and her colleagues’ study suggest that during this subduction process, the hematite and magnetite in the BIFs decomposed, producing oxygen (figure 2).
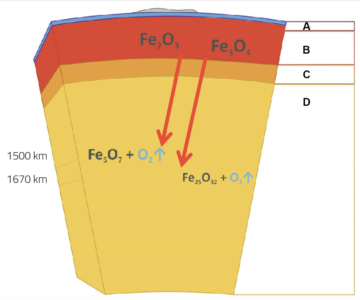
A: Crust (<100 km); B: Upper mantle (100-410 km); C: Transition zone; D: Lower mantle (660-2880 km)
Image courtesy of Elena Bykova
The quantities of oxygen involved are huge: based on the estimated rate at which BIFs are subducted, the decomposition of hematite alone in that time could have produced 8 to 10 times the mass of oxygen in the modern atmosphere. Under the conditions found in Earth’s lower mantle, Elena and her colleagues believe that this oxygen would exist in a liquid state. They suggest that over time, this has formed an enormous – and previously unimagined – reservoir of oxygen-rich fluid deep in Earth’s interior.
This fascinating idea also means that researchers will need to substantially rethink their ideas about the geochemical processes in Earth’s interior. In the presence of so much oxygen, the oxidation states of the elements in the lower mantle – and thus the chemical reactions between them – will be very different to what scientists have imagined. Furthermore, as it moves, the oxygen-rich fluid will transport other compounds, including the many trace elements present in Earth’s interior, producing a very different chemical distribution to what has previously been assumed. According to Leonid Dubrovinsky, the leader of the group that performed this study, “The effects of carbon dioxide, water and other greenhouse gases on the atmosphere are widely discussed, but the contributions of deep geochemical processes to the atmospheric composition have so far received little attention. Any global models of the past and future of Earth, including models of the evolution of our climate, will need to take into account these new findings.”
References
- Bykova E et al (2016) Structural complexity of simple Fe2O3 at high pressures and temperatures. Nature Communications 7: 10661. doi: 10.1038/NCOMMS10661
Web References
- w1 – The European Synchrotron Radiation Facility (ESRF) is one of the most intense sources of X-rays in the world. Thousands of scientists come every year to the ESRF to carry out experiments in materials science, biology, medicine, physics, chemistry, environmental science and even palaeontology and cultural heritage.
- ESRF is a member of EIROforumw2, the publisher of Science in School.
- w2 – EIROforum is a collaboration between eight of Europe’s largest inter-governmental scientific research organisations, which combine their resources, facilities and expertise to support European science in reaching its full potential. As part of its education and outreach activities, EIROforum publishes Science in School.
Resources
- To learn more about the technique of X-ray diffraction, see:
- Haddow M (2012) The new definition of crystals – or how to win a Nobel Prize. Science in School 24: 59-64.
- Cornuéjols D (2009) Biological crystals: at the interface between physics, chemistry and biology. Science in School 11: 70-76.
Institutions
Review
This article describes how, under very high temperatures and pressures similar to the conditions in Earth’s interior, previously unknown iron oxides are formed. The formation of these compounds, identified with state-of-the-art equipment, releases oxygen – a discovery that has significant implications for models of Earth’s interior and our climate.
The article could be used as part of a lesson on the internal structure of Earth, or on the evolution of climate. Suitable comprehension and extension questions include:
- What are the differences between the iron oxides mentioned in the article?
- Explain why and how temperature and pressure can affect crystal structure.
- Describe the process of subduction.
- What information can we get from X-ray diffraction?
Monica Menesini, Liceo A Vallisneri, Lucca, Italy





