Supporting materials
Instructions on how to set up the fermentation (Word document)
Instructions on how to set up the fermentation (PDF file)
Download
Download this article as a PDF

European countries produce more than half of the world’s wine – and drink a lot of it too! These hands-on activities for schools reveal the science behind the perfect wine.

The age at which it is legal to drink alcohol varies from country to country, but most teachers would agree that drinking wine during chemistry lessons is inappropriate (and potentially dangerous!). However, producing and analysing wine at school can be fun and educational. These activities, developed at the science centre Experimentaw1, invite students aged 15-18 to become vintners for a day, using analytical techniques to explore the changes that take place during the wine-making process.
Wine is produced by fermenting grape juice (which has particularly high levels of sugar) using specialised yeast cells. The sugar is converted into ethanol and carbon dioxide under anaerobic conditions:
C6H12O6 + 2 ADP + 2 Pi = 2 C2H5OH + 2 CO2 + 2 ATP
Experimentaw1 in Heilbronn is the largest informal learning and interactive science centre in southern Germany. In addition to the interactive exhibitions and science garden, Experimenta offers more than 30 laboratory-based programmes for school classes and individual pupils, from kindergarten level up to upper secondary school. These programmes address technology and all life sciences as well as providing teacher training.

The three main factors that determine the quality of the final product are sweetness, alcohol content and acid content. Using standard methods of a commercial wine laboratory, these three activities for the school laboratory explore how the quality of the starting grape juice and the must (fermenting grape juice) affect the final product. Each activity takes approximately 20-30 minutes.


Four further activities can be downloadedw2:
To supply the must used in these experiments, you will need to set up a simple grape juice fermentation at least one day in advance, using red grape juice (e.g. from the supermarket). You will also need some basic chemistry laboratory equipment, plus a vinometer for measuring alcohol content, a pycnometer (also known as a specific gravity bottle) and a refractometer. You can download a description of how to set up the fermentationw2.

The sweetness of the wine is determined by the amount of sugar remaining after fermentation, together with the total acidity of the wine. A dry wine has up to 9 g/l sugar and an acidity level that is at least 2 g/l lower than the sugar content. A medium-dry wine has a sugar content of 9-18 g/l and an acidity level that should be no more than 10 g/l lower than the sugar content. A sweet wine has 18-45 g/l of sugar. To ensure the correct balance of sugar, acidity and alcohol in the final wine, it is important to determine the starting sugar concentration; if necessary, limited amounts of sugar can be added before fermentation.
The increased density of the must (compared to water) is mainly due to fermentable sugar. Density measurements or refractometry can be used to measure the sugar content, which in Germany is expressed as the must weight and measured in Oechsle (°Oe). In the English-speaking world, the sugar content is expressed in Brix (°Bx), which represents the concentration of dissolved sugar, in weight percent (wt%).
The must weight is calculated by:
must weight = (density – 1) x 1000
Where must weight is measured in °Oe and density in g/l.
As a rough estimate, 1°Oe corresponds to 2.37 g/l sugar (i.e. about 0.237 °Bx). Therefore, the sugar concentration can be estimated as:
sugar concentration = must weight x 2.37
Where sugar concentration is measured in g/l.
The fermentation of all fermentable sugar in a solution of 100 °Oe (sugar concentration 237 g/l or 23.7 °Bx) yields approximately 100 g/l ethanol (or 10 wt% alcohol). Because ethanol has a density of 0.79 g/ml, this converts to 12.67 vol% ethanol. Thus:
alcohol concentration (in % volume) = alcohol concentration (in g/l) x 0.1267
The amount of sugar in the grape juice will determine both the alcohol content and the sweetness of the finished wine. In this activity, you will use the refractive index to estimate sugar content.
Refraction is the change in direction of light when it passes from one medium to another (e.g. from air into water). The light-scattering behaviour of a solution changes as the concentration of solutes (dissolved substances) increases. A refractometer uses this principle to determine the concentration of dissolved particles in a solution. In wine, these are principally sucrose.
Most handheld refractometers give the concentration of the dissolved substance either in Brix (°Bx), a scale defined in terms of sucrose content, or in Oechsle (°Oe). A solution of 20 wt% sucrose in water is 20 °Bx. Oechsle can be converted approximately into Brix by multiplying by 0.237.

| 20 wt% sucrose | Grape juice | |
|---|---|---|
| Must weight (°Oe) | ||
| Sugar concentration (°Bx) | ||
| Possible alcohol yield (vol%) |
The amount of alcohol obtained by fermentation depends on the sugar content of the grape juice and the alcohol tolerance of the yeast strain: most yeast strains tolerate up to 16 % alcohol. The amount of alcohol can be measured quite accurately using a vinometer, a simple device developed for hobby winemakers. It is based on the principle that the surface tension falls as the alcohol content increases.
In this activity, you will measure the alcohol content of your must.
| Alcohol content (vol%) | |
|---|---|
| Must (filtered) | |
| Wine |
Note: The alcohol content of the must is probably much lower than that of the wine. This may be because the fermentation process is not finished. It can also indicate that remaining sugar has increased the surface tension and is affecting the reading.
Fruit juices can contain several different acids, including tartaric, malic, citric and oxalic acid, in differing ratios, depending on the type of fruit. The predominant acid in wine is tartaric acid, which has a pH between 3 and 4. However, due to the complex mixture of different acids and bases, proteins and salts, the total acid content of wine cannot be estimated from the pH value alone. Instead, it is determined by titration to neutrality and expressed as total equivalent amount of tartaric acid in g/l. The acid content of wine is typically 4-8.5 g/l but can be as high as 15 g/l. It must always be considered in conjunction with the amount of remaining sugar (see ‘Determining sugar content’).
Tartaric acid (molecular weight 150 g) is a diprotic acid (containing two hydrogen atoms per molecule that can dissociate in water as protons) that can be fully neutralised with sodium hydroxide. Because 1 mol NaOH neutralises 0.5 mol tartaric acid (75 g/l), 1 ml 0.1 M NaOH neutralises 7.5 mg tartaric acid.
HOOC-CH(OH)-CH(OH)-COOH + 2NaOH → Na+-OOC-CH(OH)-CH(OH)-COO– Na+ + 2H2O
All wines contain a certain amount of acid. The winemaker is interested in the total acidity, caused mainly by tartaric acid. The total acidity is determined by titration with diluted sodium hydroxide.
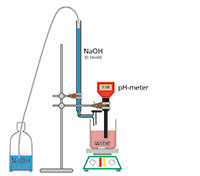
For each sample (must or wine):
Example: We used 14 ml 0.1 M NaOH to neutralise 10 ml solution. The concentration is therefore (14 x 7.5 mg/ml x 100) = 10.5 g/l acid.
| Must | Wine | |
|---|---|---|
| pH at start | ||
| Starting volume NaOH (ml) | ||
| End volume NaOH (ml) | ||
| Volume NaOH used (ml) | ||
| Concentration of acid (g/l) |
Wear safety goggles and gloves. See also the general safety note.

In activities 1-3, you analysed the three major factors that determine the quality of the final product: sweetness, alcohol and acid content. Now it is time to evaluate your product.
The author would like to thank the wine laboratory of Pfäffle GmbH in Heilbronn, Germany, for support during the development of the activities. He would also like to thank in particular Christine Dietrich and Karsten Wiese from the teacher-training college in Heilbronn for their collaboration.
Schmitt A (1975) Aktuelle Weinanalytik, Ein Leitfaden für die Praxis. Germany: Heller Chemie. ISBN: 978-3-9800498-3-2