Plastics in cars: polymerisation and recycling Teach article
What types of plastic are used to build a car? How are they synthesised and recycled? Marlene Rau and Peter Nentwig introduce two activities from the ‘Chemie im Kontext’ project.

/ iStockphoto
Many teenagers are interested in cars, which contain lots of different plastics: polymers produced from crude oil or renewable materials. An interest in cars can therefore be used to introduce the topic of plastics and polymers, for example in organic chemistry lessons.
Take your students to look at some cars. What do they already know about the plastics used to build cars? What would they like to find out? They could categorise their ideas according to different parts of the car (see Table 1, below).
Table 1: The use of plastics in cars
- Do plastics make a car more eco-friendly? (lower fuel consumption, recycling of plastic parts)
- Transparent headlights and rear lights
- Summer and winter tyres
- Strong safety belts
- Heat-resistant plastics close to the engine
- Foam in the car seats
The important point is that the components of a car have specific requirements (e.g. a seatbelt needs to be strong but flexible), which means that their constituents need specific characteristics (e.g. the material should not tear), so specific types of material (e.g. polyethylene terephthalate) need to be used. If the students do not come up with these connections themselves, ask them why a single type of plastic would not suffice to build a car.
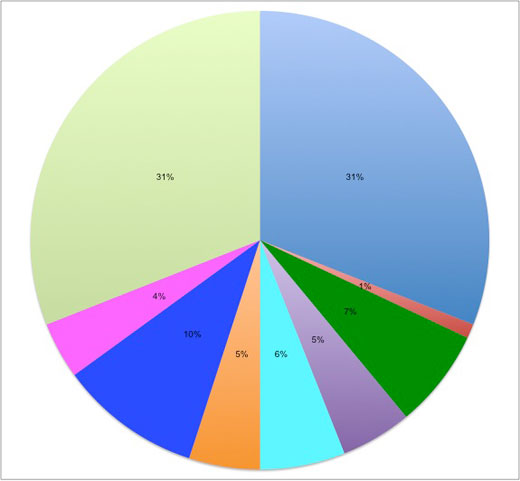
Acrylnitrile butadiene styrene terpolymer: 31%
Polyethylene terephthalate: 1%
Polycarbonate / Acrylonitrile butadiene styrene polymer: 7%
Acrylic glass: 7%
Polypropylene: 6%
Polybutylene terephthalate: 5%
Polyacetate: 10%
Polycarbonate: 4%
Polyamide: 31%
Image courtesy of the Leibniz Institute for Science and Mathematics Education
The activities in this article address two of the topics in Table 1: plastics for the rear lights and how plastics are recycled from cars. Each activity consists of a worksheet and background information. The activities are part of a longer lesson plan (see box) suitable for students aged 16+, who should work in groups of two or three. Allow one or two 45-minute lessons for each activity.
The ‘Chemistry in context’ project
‘Chemie im Kontext’ (‘Chemistry in context’) is a project co-ordinated by the Leibniz Institute for Science and Mathematics Education at the University of Kiel, Germany. Between 2002 and 2008, chemistry teachers, other science educators and representatives of the school authorities developed teaching units for chemistry education – for all grades and types of schools, linking curricular requirements to everyday contexts. Examples of the resources and recommendations for developing further materials are available onlinew1. A set of resources produced by teachers can be ordered free of charge, and four of the lesson plans are freely available online (all in German). A textbook and a teacher’s guide (in German) are published by Cornelsen Verlag.
This article is an extract from one of the lesson plans. The full lesson plan includes six different activities, one for each of the boxes in Table 1.
Polymerisation: plastic for car lights

glass
Image courtesy of BASF 1998
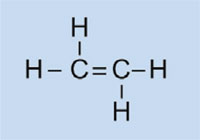
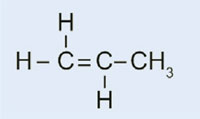
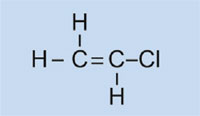
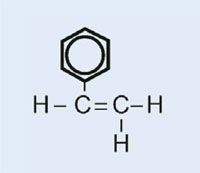
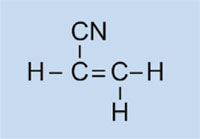
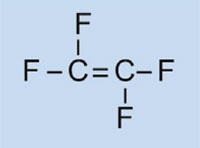
Car lights have plastic covers to keep them clean and dry, and – in some cases – to confer a colour (e.g. red for rear lights and orange for indicators). The material used must be transparent, light, colourable, easily moulded and reasonably strong. In this activity, we will synthesise the plastic used, poly(methyl methacrylate) or PMMA.
Poly(methyl methacrylate) is commonly known as acrylic glass or plexiglass, and is one of a group of plastics called polymerisates. Their common feature is that their basic monomeric units contain one or more double bonds. Under the influence of radicals (molecules with an unpaired free electron), these units undergo radical polymerisation into long-chained macromolecules.
The characteristics of the macromolecule depend on which side chains it has, which in turn depends on the monomer used. By using different monomers in the formation of polymerisate plastics, we can create plastics for different applications in cars. For example, the bulky side chains of PMMA prevent the plastic forming crystalline structures as it solidifies, which would refract light. Instead, such amorphous plastics are transparent, which makes them useful substitutes for glass: lighter, more malleable and less prone to shattering.
We can illustrate radical polymerisation with the car’s fuel tank, which is made of polyethene. Polyethene is formed from ethene (ethylene, C2H4) monomers, in a reaction initiated by dibenzoyl peroxide. When heated to 90 °C, dibenzoyl peroxide splits into two radicals. If one of these radicals binds to an ethene molecule, the ethene molecule’s double bond breaks and a new, larger radical is formed. In this way, a chain reaction begins, which only stops when two radicals react with one another.
| Monomer | Polymer |
|---|---|
| Ethene
|
Polyethene
|
| Propene
|
Polypropylene
|
| Vinyl chloride
|
Polyvinyl chloride
|
| Styrene
|
Polystyrene
|
| Acrylonitrile
|
Polyacrylonitrile
|
| Methyl ester of methacrylic acid
|
Poly(methyl methacrylate)
|
| Tetrafluoroethylene
|
Polytetrafluoroethylene
|


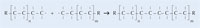
Figure 2: The steps involved in polymerisation
Images courtesy of the Leibniz Institute for Science and Mathematics Education
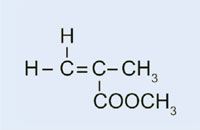
In our experiment, we will use dibenzoyl peroxide to initiate a similar process: instead of using ethene to form polyethene, we will use methyl 2-methylpropenoate to produce poly(methyl methacrylate).
Student worksheet 1: synthesising a transparent polymer
Materials

Image courtesy of the Leibniz
Institute for Science and
Mathematics Education
- Methyl 2-methylpropenoate (also known as methyl methacrylate; C5H8O2)
- Dibenzoyl peroxide (C14H10O4)
- Sudan Red dye
- Water
- Acetone (propanone, C3H6O)
Equipment per group
- A balance
- A heating plate
- A test tube
- A beaker
- A watchglass
- A spatula
- A pipette
- The aluminium holder from a tea light
Safety note
Use gloves, safety goggles and work under a fume hood. Methyl 2-methylpropenoate, dibenzoyl peroxide and acetone are flammable; acetone is also an irritant. All three must be used with care. Remaining Sudan Red must not be disposed of in waste water.
See also the general safety note.
Procedure
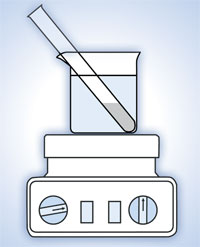
- Weigh 0.2 g of dibenzoyl peroxide into the test tube.
- Add 10 ml of methyl 2-methylpropenoate.
- To colour the resulting plastic, add a little Sudan Red (enough to cover the tip of the spatula).
- Place the tube into a beaker full of water at 90 °C and place it on the heating plate; see Figure 3, below.
setup
Image courtesy of the Leibniz
Institute for Science and
Mathematics Education
The reaction will take about 20 minutes, after which the mixture should be visibly viscous. In the meantime, read the information under the heading ‘Polymerisation: plastic for car lights’ and work out the reaction mechanism for the radical polymerisation of the methyl 2-methylpropenoate.
- Pour the solution into the aluminium holder from a tea light.
If the plastic starts to solidify in the test tube, you can dissolve it again with acetone. You can then continue the experiment as described, allowing additional time for the acetone to evaporate.
- Cover the aluminium holder with the watchglass to keep the plastic warm and allow it to harden faster.
- Let the poly(methyl methacrylate) solidify for 24 h, then remove it from the aluminium holder.
How would you test the properties of your plastic and compare them to those of glass?
Recycling plastics from cars

of different fuels; from left to
right: heating oil; plastic
waste such as olefins; lignite.
Click on image to enlarge
Image courtesy of the Leibniz
Institute for Science and
Mathematics Education
In this activity, students first learn about how plastics from cars can be recycled, then try their hand at recycling: transforming a plastic bottle into a piece of shaped plastic.
What happens when we have finished with a car? We may think of piles of rusting cars and old tyres, destined for landfill, but in fact many car parts are recycled to recover valuable resources, especially metals.
The plastics in a car can also be recycled, in three ways: as parts, chemical components or fuel.
- Cars may be repaired using old plastic parts such as bumpers. As the parts get older, however, their characteristics change, making them inappropriate for reuse: solar radiation, for example, makes most plastics brittle. Fortunately, some plastic parts can be melted down and reshaped into articles for which the quality requirements are lower, such as fence posts or garden benches.
- Through chemical processes, some polymers can be split up into their monomeric components, which are then available for new syntheses. Plastics can also be used to produce other resources for the chemical industry; for example, one tonne of certain types of used plastic gives about 600 kg of methanol, which is both an important resource for the plastics industry and used in fuel cells to release energy.
- Shredded plastics can be directly used as fuel, substituting oil and coal, for example in waste-to-energy plants. They can also replace coke used for iron production in furnaces (see Figure 4).
Student worksheet 2: recycling plastic in the classroom
In this activity, you will recycle waste plastic from a bottle into shaped pieces of plastic. What you do with these is limited only by your imagination: key rings, pendants, Christmas tree decorations

Image courtesy of hippokrat /
iStockphoto
Materials per group
- A Bunsen burner
- A tripod with a wire mesh
- Biscuit cutters in different shapes
- Aluminium foil
- A knife
- Plastic waste (preferably PET bottles for mineral water)
- A selection of dyes
Safety note
Use safety glasses and work using the fume hood. Do not allow the flame to come into direct contact with the plastic. Be careful not to cut yourself.
See also the general safety note.
Procedure
- Use the knife to shred the plastic waste as small as possible.
- Cover the bottom and sides of biscuit cutters with foil and fill them to a depth of about 0.5 cm with shredded plastic waste. If you wish, try adding a small amount of dye to the plastic waste.
- Place the cutters on the wire mesh above the Bunsen burner and heat them slowly until the plastic melts.
- Once it has cooled, take out the plastic and remove the foil.
Compare the characteristics of the plastic before and after recycling. What conclusion can you draw about plastic recycling?
In your group, discuss the three recycling methods described under the heading ‘Recycling plastics from cars’ and compare possible applications. Reflect on the experiment you have carried out: what are possible applications for your plastic?
Studying plastics with X-rays

At the European Synchrotron Radiation Facility (ESRF)w2, high-performance fibres such as the plastic Kevlar® have been studied for more than a decade. Five times stronger than steel, weight for weight, Kevlar is used in bicycle tyres and body armour, racing sails and mooring lines.
Much of ESRF’s work on high-performance fibres involves their skin-core morphology: the differences in structure between the outer layers of the fibre and its centre. These differences can influence the fibre’s mechanical properties, so understanding the skin-core morphology may enable the fibres’ properties to be tailored during the manufacturing process.
ESRF’s microfocus X-ray beams are routinely used for new studies of these remarkable materials, for both academic and industrial research. This is because no other techniques provide similar information without sectioning the fibre and potentially altering its internal microstructure. For more information, see Capellas Espuny, 2009.
ESRF is a member of EIROforumw3, the publisher of Science in School.
References
- Capellas Espuny M (2009) A new look into fibre-reinforced composite materials. ESRF News 50: 12-13. www.esrf.eu/UsersAndScience/Publications/Newsletter
Web References
- w1 – To learn more about the project (in English and German) and download the resources (in German), see the Chemie im Kontext website: www.chik.de
- w2 – An international research centre in Grenoble, France, ESRF produces high-brilliance X-ray beams, which serve thousands of scientists from all over the world every year. For more information, see: www.esrf.eu
- w3 – To find out more about EIROforum, see: www.eiroforum.org
Resources
- To learn more about the ‘Chemie im Kontext’ project, see:
- Parchmann I et al. (2006) Chemie im Kontext: a symbiotic implementation of a context-based teaching and learning approach. International Journal of Science Education 28(9): 1041-1062
- Nentwig P et al. (2007) Chemie im Kontext: situated learning in relevant contexts while systematically developing basic chemical concepts. Journal of Chemical Education 84: 1439
- For a drama activity about the radical polymerisation of ethene to polyethylene in class, see:
- Sturm B (2009) The drama of science. Science in School 13: 29-33.
- To learn about research into biodegradable plastics, see:
- Bradley D (2007) Plastics, naturally. Science in School 5: 66-69.
Review
Chemistry is usually seen by students as distant and dangerous, but we are surrounded by it and it plays a major part in the improvement of our quality of life. Activities such as the ones in this article, which use everyday materials, can help to increase the public appreciation of chemistry and encourage interest in it among students.
These activities could be used in organic chemistry lessons and also in biology, to discuss the importance of recycling. Although the authors suggest the activities for students aged 16+, they could also be used with younger students (aged 14+).
Mireia Guell Serra, Spain