Household chemistry: solvents and stain removers Teach article
Clearing up chemistry: household products like nail polish remover and laundry detergent can be used to demonstrate chemical concepts like intermolecular forces and redox reactions.
Tying science teaching to students’ personal experiences and everyday life can help them to engage with and understand chemical concepts. This article presents simple experiments using products that can be found either at home or in the supermarket. The activities cover a variety of ideas in chemistry: intermolecular forces, redox reactions, and the properties of a solvent.
All the proposals are presented to be adaptable to different educational levels, from 13 to 18 years, and can be used with different teaching approaches (e.g., teamwork, case-based learning).
Activity 1: Investigation of molecular forces with acetone from nail polish remover
Experiment 1: Investigating the solvent acetone
Acetone is the solvent usually used to remove nail polish, although sometimes alcohol (ethanol) is used. The boiling points of these two liquids are below that of water. This attribute makes them volatile liquids.
Safety note
Wear a lab coat, gloves, and safety glasses. Acetone is highly flammable. Keep away from heat sources, sparks, and open flames. Causes irritation to the eyes. Prolonged exposure may cause skin dryness or cracking.
Materials
- 2 ml each of water, acetone, and ethanol
- Balloons of different colours, one for each liquid
- 600 ml or larger beakers, one for each balloon
- Very hot water (enough to half fill the beakers)
Procedure
- Heat the water to about 80°C in each beaker. Or use a kettle to heat water and half fill the beakers.
- In each balloon, put about 2 ml of one of the liquids. Tie the balloon securely.
- Place the balloons in hot water. They will swell, depending on the ease of volatilization of the liquid within.

Image courtesy of the authors

Image courtesy of the authors
- Explain the results with reference according to the strength of intermolecular forces between the solvent molecules.
An alternative is to place a few millilitres of each liquid in different test tubes. Different coloured balloons are placed in each tube and the tubes are soaked in hot water. More volatile liquids inflate balloons more.

Discussion
Discuss the following questions with your students to explore the key concepts:
- Which of the balloons swells faster?
- In which of the balloons do you conclude that the intermolecular forces in the solvent are weaker?
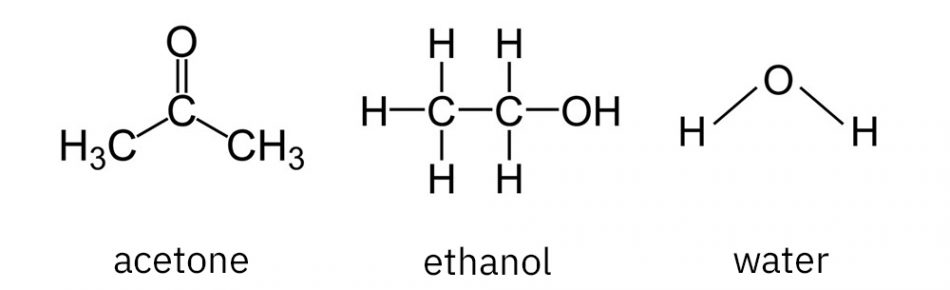
- By comparing the molecular structures of the three substances (acetone, ethanol, and water) which molecules form hydrogen bonds?
Explanation
In volatile liquids, the molecules have weaker bonds between each molecule than those between water molecules.
In this case, the weaker bonds may be explained by weaker polarity of the molecules. This is the case for very weak interactions, such as London forces (induced dipoles), which are part of van der Waals interactions and hydrogen bonds.
Experiment 2: Demonstrating three properties of acetone
This demonstration involves a few drops of acetone. For the demonstration to be more effective, it’s better to lower the lighting in the room.
Safety note
Wear a lab coat, gloves, and safety glasses. Acetone is highly flammable. Keep away from heat sources, sparks, and open flames. Causes irritation to the eyes. Prolonged exposure may cause skin dryness or cracking.
Materials
- PVC tube, about 2 m long and 2 cm in diameter, arranged in a spiral
- Four laboratory clamp stands
- Wire
- Acetone
Procedure
- Space the laboratory clamps about 40 cm from each other to form a square.
- Place the PVC tube in a downward spiral around the clamps and fix with wire.
- Place a small burning candle at the end of the tube.

Image courtesy of the authors
- Pour between 1 and 2 ml of acetone into the top of the tube.
- Observe a blue flame running through the tube after a few seconds.
Discussion
Discuss the following questions with your students to explore the key concepts:
- Why does the flame go up the tube?
- The letters at the bottom of the assembly represent acetone volatile density combustion. How does this experiment demonstrate these three properties of acetone?
Explanation
Firstly, acetone is volatilized, then acetone vapour, which has a higher density than air (acetone: ρ = 2.4 g/l; air: ρ = 1.1 g/l, under standard conditions), descends through the tube and is ignited by the flame due to the mixture of air and acetone vapour created inside the tube.
Activity 2: Investigating redox chemistry with destaining agents
Safety notes
Wear a lab coat, gloves, and safety glasses. Follow the safety advice on the packaging for all cleaning products.
Sodium perborate is toxic by ingestion and may cause respiratory irritation if inhaled. Avoid contact with the eyes. If this occurs, rinse with plenty of water.
Sodium percarbonate, under normal use conditions and in its original form, is not dangerous, but it can cause irritation if inhaled. In case of eye contact, rinse with plenty of water.
Sodium hydroxide is a strong base. In case of skin contact, wash immediately with plenty of water. In case of eye contact, rinse carefully with water for several minutes (removing contact lenses first) and seek medical attention.
Materials
- Test tubes
- Spatula
- Dropper
- 3% hydrogen peroxide (H2O2)
- Sodium perborate (NaBO3)
- An ‘active oxygen’ bleach tablet based on sodium percarbonate,
such as Neutrex - A solution of the antiseptic povidone iodine (often sold under the tradenames Betadine and Topionic)
- 1 M sodium hydroxide (NaOH)

Tablets containing 5–15% sodium percarbonate.

Procedure
- Prepare three tubes:
Tube 1: 1 M NaOH (1 ml) + H2O2 (1 ml)
Tube 2: water (1 ml) + a spatula tip of sodium perborate
Tube 3: water (1 ml) + a spatula tip of active oxygen tablet
- Gently heat tube 2.
- Add 5 drops of povidone iodine to each tube.
- Write down what you see happening in each test tube.
Discussion
Discuss the following questions to explore the key concepts:
- Explain the effect of adding sodium perborate to the second test tube
- Explain the effect of adding the active oxygen tablet(with sodium percarbonate) to the third test tube
- ‘Green detergents’ contain sodium percarbonate. Give a reason why these products are considered better for the environment than those with sodium perborate. Hint: consider the temperature.
Explanation
Povidone iodine is a water-soluble chemical complex composed of iodine and polyvinylpyrrolidone.

Image: Modified from Vaccinationist/Wikimedia, Public Domain
The iodine molecule, which gives the brown colour to the povidone iodine solution, reacts with H2O2 to give HI, which is colourless.
In each tube, the formation of gas bubbles is observed. This gas is oxygen formed through the decomposition of H2O2 as it reacts with the iodine.

Tube 2: sodium perborate
Tube 3: bleach tablet containing sodium percarbonate
Image courtesy of the authors

Image courtesy of the authors
Tube 1
Oxidation: H2O2 (aq) + 2 OH− (aq) → O2 (g) + 2 H2O (l) + 2 e−
Reduction: 2 e− + I2 (aq) → 2 I− (aq)
Complete reaction: H2O2 (aq) + I2 (aq) + 2 OH− (aq) → O2 (g) + 2I− (aq) + 2H2O (l)
We add a few drops of sodium hydroxide solution to make the medium alkaline. Otherwise, the reaction will not take place.
Tube 2
Firstly, a decomposition reaction of the perborate ion occurs when it dissolves in water and then hydrogen peroxide that is formed reacts with the povidone iodine complex. Sodium perborate can act as a bleach and generate oxygen in an aqueous solution from the perborate molecule only when it reaches a temperature equal to or greater than 60°C.
NaBO3 (aq) + H2O (l) → NaBO2 (aq) + H2O2 (aq)
Then hydrogen peroxide reacts with iodine as before:
Oxidation H2O2 (aq) + 2 OH− (aq) → O2 (g) + 2 H2O (l) + 2e−
Reduction 2e− + I2 (aq) → 2I− (aq)
Complete reaction: H2O2 (aq) + I2 (aq) + 2 OH− (aq) → O2 (g) + 2 I−(aq) + 2 H2O (l)
Tube 3
When sodium percarbonate is dissolved in water, it decomposes into two substances: sodium carbonate (a surfactant that improves its effectiveness as a detergent) and hydrogen peroxide.
Neutrex is a strongly alkaline detergent that contains sodium percarbonate, so the results are the same as those for the second test tube. The alkaline nature of this substance means it is not necessary to add a few drops of sodium hydroxide solution for the reaction to take place in this test tube.
Green detergents contain sodium percarbonate, which works in cold water. This means that heating is not required, which makes the washing process more sustainable.
Na2CO4 (aq) + H2O (l) → Na2CO3 (aq) + H2O2 (aq)
Then hydrogen peroxide reacts with iodine as before.
Acknowledgements
The experiments in the article are developed by the authors or they have been modified from procedures reported by other authors[1–3] with changes to the products and the context under which the experiments are performed.
All experiments have been tested in class by students in a secondary school and presented as a workshop at the congress of the French Union of Physics and Chemistry Teachers in Bordeaux 2018 (UdPPC) and at the congress of the Belgian Association of Physics and Chemistry Teachers (ABPPC), Louvain la Neuve (2017).
References
[1] Schwedt G (2003) Experimente mit Supermarktprodukten: Eine chemische Warenkunde. Wiley-VCH, Weinheim. ISBN: 3-52732-450-X
[2] Andersen E, Brown A (2012) The effect of heat: simple experiments with solids, liquids and gases. Science in School 24: 23–28.
[3] Segura M, Corominas J (2020) POP chimie. Le Bup physique chimie 114: 271–285.
Resources
- Watch a video about the ingredients in nail polish.
- Read about the science of hair dye: Guenard R (2015) Colour to dye for. Science in School 32: 10–13.
- Try some fun experiments with laundry detergents: Soler ML (2019) Which laundry enzymes work best? Science in School 46: 34–39.
- Discover the chemistry we can learn using tea: Prolongo M, Pinto G (2021) Tea-time chemistry. Science in School 52.





