Supporting materials
Download
Download this article as a PDF

When we sleep, are we just passively recovering from a hard day, or is there something more going on? Angelika Börsch-Haubold considers the implications of some intriguing research – was her grandmother right all along? Test the scientists’ conclusions for yourself!

My grandmother used to say that a good night’s sleep is essential for learning. She even insisted that the sleep before midnight was best. Children are usually happy to go to bed when tired but most teenagers see it as part of their emancipation from parental interference to stay up late. And our society seems to approve: today, we sleep 20% less than our ancestors did because we push ourselves to work longer hours and to have evenings full of social activities (Hargreaves, 2000).
Everybody knows that our own cognitive performance is impaired after a late night or even after regularly losing small amounts of sleep. Fatigue is estimated to be an important factor in one-third of traffic accidents and to be the number one cause of fatal crashes in 18- to 25-year-olds. But sleeping too little puts not only your own life at risk. In an anonymous survey in San Francisco, over 40% of hospital staff admitted causing the death of at least one patient by making a fatigue-related mistake.
A simple indicator of sleep deprivation is the urge to fall asleep during the day. This happens to pupils during lessons, to students over their textbooks, to travelling business people, or to elderly people in front of the TV. So, sleep deprivation is a widespread phenomenon and apart from its potentially fatal consequences, this impinges strongly on our ability to remember and hence to learn.
In the past few years, scientists have discovered that there is more to sleep than a simple refreshing of our nerves. The process of learning is actively taken up by our brain during sleep (Huber et al., 2004), which is essential for the formation of long-term memory. The first memory that is built when we learn a new task is susceptible to interference. After a certain time, an automatic process called memory consolidation sets in, which stabilises that memory. Memory consolidation continues during sleep, but then leads to the additional effect of memory enhancement. Thus, our brain performs better after an afternoon nap and much better after a full night of sleep (Stickgold, 2005). Also, when we are searching for the solution for a tricky problem, our brain continues to work on it while we are sleeping. As a result, we may experience a sudden understanding of a rule, an insight, that is sometimes even triggered by a dream. Two experiments that measure the effect of sleep on memory consolidation and on sudden insight are described below.
The hypothalamus, as the central regulatory organ of the autonomic nervous system, controls the circadian rhythms (body clock) of our body temperature, hormone release, appetite and sleep. It contains a neuronal switch that regulates ‘wake nerves’ and ‘sleep nerves’. A sudden transition to the sleeping mode (‘falling’ asleep) means that sleep nerves fire to inhibit wake nerves. The switch is stabilised by a third group of neurons, otherwise we would frequently wake up at night (Saper et al., 2005). To picture the neuronal mechanism underlying sleep, imagine three children on a see-saw. One sits on either side of the beam bouncing up and down – the switch. The third child sits on top of the axis. When it shifts its weight to one side of the beam, it effectively stops the motion – the stabiliser.
This dynamic system generates the different stages of sleep that have been known for some time: rapid eye-movement sleep (REM) in which dreaming occurs, and non-REM sleep with the light sleep stages I and II and the deep sleep stages III and IV. The pattern of a night of sleep is characterised by 90-minute cycles composed of deep sleep and light sleep phases. An initial long period of stage III and IV sleep is followed by a short period of REM and stage I and II sleep. As the night progresses, the deep sleep phases become shorter and the dreaming phases longer.
The physiological function of sleep seems to be two-fold. First, non-REM sleep is a period of low metabolic demand in which the adenosine triphosphate (ATP) energy stores, used up while we are awake, are replenished. The degradation product of ATP, adenosine, acts as a physiological sleeping agent by directly activating the sleep-promoting neurons. Second, sleep plays a key role in neural plasticity. During sleep, significant connections between neurons are reinforced while accidental connections are eliminated. All the different stages of sleep have been implicated in sleep-dependent learning.

The effect of sleep on learning is easiest to quantify when measuring unconscious learning. This can be done with experiments to test motor skills, such as finger-tapping on a keyboard or tracing a line displayed on a computer screen, or by testing perceptual skills, such as differentiating between diagonal bars in the foreground against horizontal bars in the background.
In the finger-tapping task, subjects repeatedly type a numeric sequence, for example 4-1-3-2-4, as fast as possible (see experimental protocol). They get better over the first five minutes of practice, after which they approach an asymptote of about 60% improvement over twelve 30-second trials (Stickgold, 2005). Retesting 4 to 12 hours later the same day gives no further improvement. After a night of sleep, speed and accuracy of tapping is enhanced on average by 20%. After another two nights, a further increase of 26% is measured. These data clearly demonstrate the process of memory enhancement through sleep.
Interestingly, sleep only helps if we do not learn too much at a time using the same type of memory. During the finger-tapping experiment, when a second, unrelated sequence is learned immediately after the first training session, only the tapping of the second sequence improves after sleeping (Walker et al., 2003). Different time settings of training and retesting show that memory undergoes the first stabilisation phase of consolidation within 10 minutes to 6 hours after learning and only then becomes resistant to interference from a competing memory. However, brief periods of rehearsal (as in the retest situation) return the memory to a labile state in which it again becomes vulnerable to interference from a competing motor pattern in need of consolidation.
The scientific evidence for sleep-dependent consolidation of our memory for events (what happened yesterday) and facts (name of a new colleague at work) is weaker. However, in subjects asked to memorise nonsense syllables or to find word-pair associates, early night sleep (rich in deep sleep) supports the stabilisation of this ‘declarative memory’ (Stickgold, 2005).
Sudden insight is a form of complex cognitive learning. A prominent example of how sleep can trigger insight is given by the discoverer of the double helix, James Watson. When working in early 1953 on the base-pairing rule, an important prerequisite for solving the structure of DNA, he thought that he had found a way to pair the four bases adenine, guanine, cytosine and thymine as like-with-like structures via hydrogen bonds. Soon his crystallography colleagues pointed out that this model could not be correct for two reasons. First, Watson was told that guanine and thymine were more likely to be in the keto-configuration (contrary to textbook drawings showing the enol-configuration); second, he was reminded of Chargaff’s Rule, that adenine equals thymine and guanine equals cytosine in the overall distribution in DNA.
Equipped with this new knowledge, Watson still did not find the right solution that day, although he cut out cardboard models of the bases. However, of the next morning he writes: “Suddenly I became aware that an adenine-thymine pair held together by two hydrogen bonds was identical in shape to a guanine-cytosine pair […] All the hydrogen bonds seemed to form naturally […] Chargaff’s rule then suddenly stood out as a consequence of a double-helical structure for DNA” (Watson, 1980). The Watson-Crick double helix was born.
Wager and co-workers have devised a test that determines exactly when such abstract insight occurs in the time course of learning (experimental protocol; Wagner et al., 2004). Subjects apply a standard algorithm (consisting of two simple rules) for reducing an eight-digit sequence to a final answer. They do not know that a simple shortcut exists. The percentage of subjects who discover this shortcut or ‘hidden rule’ when they are retested is 22% in the awake group versus 60% in the sleep group. Thus, the discovery of a complex rule, one of the most sophisticated human cognitive activities, becomes much easier after a night of sleep even if the test person did not know that there was a rule to be discovered.
Recent research on sleep and learning has put my grandmother’s saying on a sound scientific footing. Combine this with the clear indicators of widespread sleep deprivation in our society, and we realise that it is time to re-evaluate our sleeping habits. As we profit for an entire lifetime from efficient learning strategies, an insight into the sleep requirements of our brain should teach us how to learn, work and play in harmony with – rather than against – our neurophysiological background. So, sleep on it.
For the finger tapping task, please download the pre-programmed Excel worksheet FingerTap.xlsw1. Before the spreadsheet is used, copy it and save the copy under the name of the student learning the task.
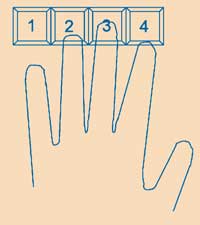
Two students work together. One of them, who needs to be right-handed, puts four fingers of his/her left hand onto the keys 1 to 4 of the computer keyboard, see figure. Starting in the column ‘run 1’, the subject must repeatedly tap the five-figure target sequence shown at the top of the sheet as quickly and as accurately as possible. The typed sequence is not displayed in the cells. After each sequence, the student presses the enter key with his/her right hand to move the cursor one cell down. The partner measures the time.
Each run is performed for 30 seconds, followed by a 30 second rest; the training phase consists of 12 runs (one in each of the ‘train’ columns labelled ‘run 1’ to ‘run 12’). The retest with 6 runs (‘retest’ columns ‘run 1’ to ‘run 6’) under the same conditions is performed either 6-8 hours later on the same day (no sleep group) or the next day after a full night’s sleep (sleep group).
The first sheet of the Excel spreadsheet is programmed to count all correct sequence entries and to give the result in the row entitled ‘score’ (row 56). The second sheet automatically analyses the data, plotting the run number against the score result and comparing the training results with the retest results. To quantify the difference between the sleep and the no-sleep group, the mean number of correct sequences of the training phase is compared with the mean of the retest; the mean values for each student are automatically calculated in the spreadsheet.
Examples of completed spreadsheets can be downloadedw1.
Students are taught a standard algorithm for reducing a given eight-digit sequence (composed of the digits 1, 4, and 9) to a final solution (see figure). They must apply two simple rules:

After the first response, comparisons are made between the preceding result and the next digit (i.e. between the circled digits; the arrow points to the correct result). The seventh response indicates the final solution; the time needed to solving the sequence should be recorded.
What the students do not know is that there is a ‘hidden rule’: the strings are generated in such a way that the second response of each trial coincides with the final response. Insight into this hidden rule sharply reduces the time needed to reach the final response for a string.
Before the experiment starts, students perform without mistakes on ten practice sequences (that do not obey the hidden rule) to ensure their understanding of the ‘same’ and ‘different’ rule. A Word document with practice and experiment sequences (NumberRed.doc) can be downloadedw1.
The experiment consists of an initial training phase (three task blocks each of 30 sequences), a period of 8 hours of sleep or wakefulness and retesting (ten task blocks). At the end of the experiment, the percentage of subjects who recognised the hidden rule is calculated and the sleep group is compared with the no-sleep group. In the original publication, subjects who recognised the hidden rule reduced their solution time per string abruptly from 8.7 to 2.4 seconds.
When preparing the experiment, make sure that students do not suspect that there might be a shortcut to the solution. If a student discovers the hidden rule during initial training, his or her data should be excluded from final analysis.
With thanks to Robert Stickgold (Harvard Medical School, Boston, MA, USA) and Ullrich Wagner (Universität Lübeck, Germany), who conducted the research reported here, for their comments on the design of the classroom activities.
w1 – All the materials needed for the exercises can be downloaded here:
This is an article that will capture the interest of pupils and teachers. It sets some complex scientific research and theory in simple terms. The article and experiments may be appropriate as part of the new GCSE courses (ages 14-16) in England, but could also be used with pupils of other ages and in other countries.
Clara Seery, UK
Download this article as a PDF