Solving crimes with chemistry Teach article
Use a common chemical technique from the field of forensics to reveal fingerprints in the laboratory.
domnitsky/Shutterstock.com
Detecting fingerprints has been a key aspect of criminal investigations for over 100 years. It is an important forensic tool for two reasons: first, fingerprints are unique (even identical twins have different patterns), and second, fingerprints do not change over time: if you burn or cut your fingertip, the pattern will re-form as it was before. At a crime scene, ‘patent’ fingerprints are easy to detect. These are visible prints left behind by blood-covered fingers, for example. ‘Latent’ fingerprints, however, pose more of a problem. These are invisible prints left by the natural oils and sweat on our skin.
Over time, forensic scientists have developed ways to visualise latent fingerprints. The oldest technique is the powder method, whereby the fingerprint is carefully dusted with dry powder using a fine brush. The powder adheres to the moisture and oil in the fingerprint, making it visible. There are now hundreds of powders with different compositions depending on the surface they are used for. In general, the powders contain pigments to provide contrast, and binders to help them adhere to the fingerprint. The powder method is still used today because it works on almost all non-absorbent surfaces, such as glass.
Another common visualisation technique is cyanoacrylate fuming. This method is used by the police not on site but in the laboratory. Cyanoacrylates are the molecules found in superglue, so the technique is also referred to as the superglue method. Like the powder method, cyanoacrylate fuming is suitable for detecting fingerprints on non-absorbent surfaces. The fuming is carried out in a developing chamber. The cyanoacrylate forms a vapour that adheres to the fingerprint after polymerisation reactions, creating a visible white print.
After development with cyanoacrylate, powders or stains can be applied to the fingerprint to improve the contrast and make it more visible. Fingerprints are often stained with crystal violet solution, which turns them purple.
The activity outlined in this article enables students to detect their own fingerprints using the superglue method. After making their fingerprints visible, students add fluorescent dye to the prints to add contrast. This procedure allows students to experience a safe alternative to staining fingerprints, since crystal violet cannot be used in the classroom due to its toxic and carcinogenic effects. We recommend that students work in small groups of three. Each part of the activity takes approximately 30 minutes.
Part 1: Visualising latent fingerprints
In this procedure, students detect latent fingerprints using the superglue method to learn about polymerisation reactions. The superglue method involves evaporating superglue in a closed chamber. We used a Tic Tac® box for the chamber and replaced the lid with a reinforced plastic lid to ensure that the vapour could not escape. Alternatively, you could use a Tic Tac® box with a Petri dish for the lid, or exchange the box for a drinking glass, and again use a Petri dish for the lidw1. Note that this equipment should not be used for other purposes after the experiment, so it needs to be disposed of.
Safety note
Wear a lab coat, gloves and safety glasses. Be careful when handling superglue as it can glue skin – such as fingers and eyelids – together in a few seconds. Cyanoacrylate vapour is a respiratory tract irritant, so the activity must be carried out in a well-ventilated room. If your school laboratory has access to a fume hood, it is advised to carry out step 6 onwards inside the fume hood.
Materials
- Large Tic Tac® box (8 cm x 4 cm x 1.5 cm)
- Reinforced plastic lidw1
- Heating plate
- Tweezers
- Scissors
- Kitchen roll
- Cellulose fleece (e.g. disposable nappy liners)
- Aluminium foil
- Ethanol
- Superglue (liquid glue works best)
Procedure

for the superglue method
Rachel Fischer
- Using scissors, cut a piece of fleece measuring approximately 10 cm x 15 cm and place it inside the Tic Tac® box.
- Cut a piece of aluminium foil measuring approximately 4 cm x 4 cm. Add some ethanol to a piece of kitchen roll, and use it to rub the surface of the foil to remove any dirt.
- Choose one person in the group to create a fingerprint on the aluminium foil. The person should rub one finger on his or her forehead or nose to pick up oil and sweat, then carefully place the fingertip on the piece of foil for two seconds.
- Using tweezers, place the aluminium foil inside the box.
- Add ten drops of superglue to the fleece. The fleece increases the surface area for evaporation and prevents the superglue from reaching the bottom of the box and hardening.
- Close the lid of the box and place the box on the heating plate.
- Set the heating plate to 75°C. If the temperature rises above 100°C the box will melt, so ensure that the temperature remains at around 75°C (see figure 1). Note that using glassware rather than the Tic Tac® box would eliminate this risk.
- As soon as the fingerprint is visible, remove the box from the heating plate. Wait ten seconds, and then remove the aluminium foil with the tweezers. To prevent the superglue from escaping, open the lid only briefly.
- If the fingerprint is not yet fully developed, apply another ten drops of superglue to the fleece and repeat the procedure.
- Repeat the procedure for each student in your group, and compare your fingerprints. Note that after five repetitions, you will need to replace the fleece.
- Once you have completed the procedure for all group members, place the box under a fume hood with the lid open. After a few minutes, the box can be disposed of in the domestic waste.
Discussion
Discuss some of the following questions as a class:
- What did you see when you turned on the heating plate?
- What does the fingerprint look like after the experiment?
- Why does the fingerprint turn white, while the rest of the aluminium foil remains the same?
- What type of reaction occurs when the superglue adheres to the fingerprint?
- What are the reactants and products in this chemical reaction?
- How can superglue glue your hands and eyelids together within seconds?
Explanation
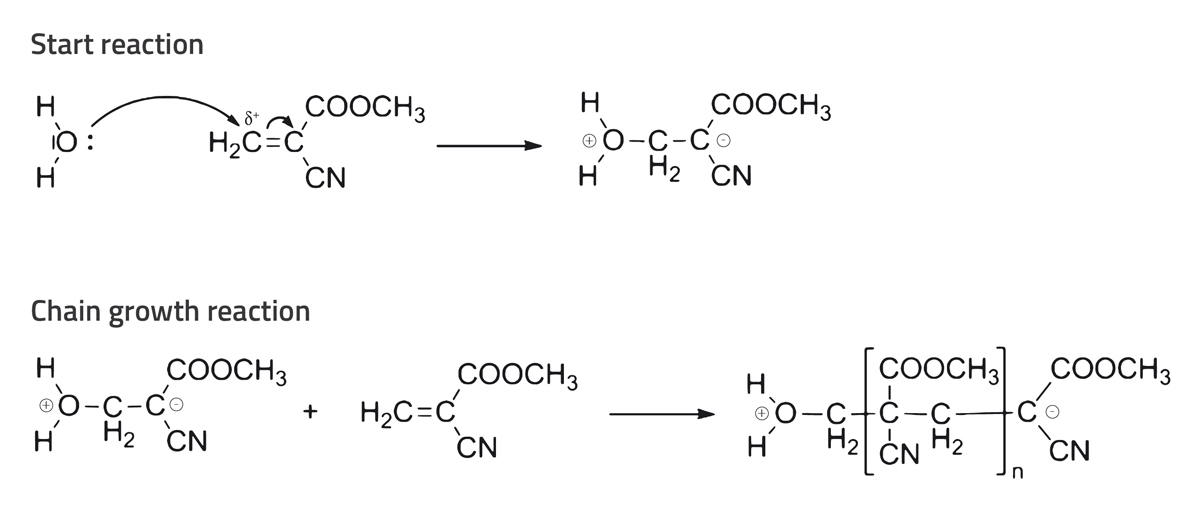
A few minutes after the heating plate is switched on, gaseous superglue rises from the fleece. This superglue vapour adheres to the fingerprint, leaving behind a white trace (see figure 2). This reaction occurs because the cyanoacrylate esters in superglue polymerise on contact with molecules in the fingerprint (e.g. water, fatty acid, amino acids). In this process, a cyanoacrylate ester (most commonly an ethyl cyanoacrylate monomer) becomes a molecular chain (an ethyl cyanoacrylate polymer; see figure 3). At room temperature, an ethyl cyanoacrylate polymer is solid and white, and it is produced in the area where you left your fingerprint. This method works only on dry objects. If the entire piece of aluminium foil were wet, the water would make polymerisation take place on the whole surface of the foil.

Rachel Fischer
Rachel Fischer
Part 2: Making fingerprints fluoresce
In this part of the activity, students stain the fingerprints from part 1 using a handmade dye. To create the dye, students dissolve ink from highlighter pens in ethanol. Students learn about the electromagnetic spectrum and fluorescence, as well as polar and non-polar molecules.
Safety note
Wear a lab coat, gloves and safety glasses – ideally UV protection glasses. Do not look directly towards the UV lamp or shine the light into someone’s eyes.
Materials
- UV lamp (λ = 395 nanometres)
- Tweezers
- Three Petri dishes
- Three pieces of filter paper
- Stabilo Boss® highlighter pens in yellow, orange and pink
- Aluminium foil with superglue fingerprints from part 1
- Ethanol
- Cardboard box
- Hair dryer (optional)
Procedure
- Take a piece of filter paper and colour the surface with the yellow highlighter. Repeat the process with the other two pieces of filter paper and two highlighters (see figure 4).
- Place each piece of paper in a separate Petri dish. Add ethanol to each dish until the paper is completely covered (see figure 5).
- After ten minutes, remove the filter papers from the Petri dishes using tweezers and dispose of them in your domestic waste.
- Choose three of the aluminium foil fingerprints from part 1, and place one in each of the solutions in the Petri dishes (see figure 6).
- Wait five minutes and then remove the aluminium foil from the solutions using tweezers. Leave the foil to dry (the ethanol will evaporate) or use a hair dryer to speed up the process.
- Observe the dyed fingerprints in a dark cardboard box or in a darkened room under UV light (see figure 7).

Rachel Fischer

Rachel Fischer

Rachel Fischer

Rachel Fischer
Discussion
Discuss some of the following questions as a class:
- What happens when you pour ethanol over the coloured filter paper?
- For this experiment to work, the dye must contain both polar and non-polar components. Why?
- What does the fingerprint look like under UV light?
- Why does the fingerprint fluoresce under UV light?
Explanation
When the highlighted filter paper comes into contact with ethanol, the fluorescent dye from the highlighter pen diffuses out from the paper into the solution. To dissolve in ethanol (a polar molecule), the dye must contain some polar components. To stain the superglue fingerprint (comprising non-polar ethyl cyanoacrylate polymers), the dye needs to have non-polar components, too. A highlighter from Herlitz®, for example, would not stain the fingerprints, because the dye (pyranine) is polar (Ducci & Oetken, 2018).
Once the ethanol evaporates off the aluminium foil and the fingerprint is stained with the dye, the fingerprint will fluoresce when irradiated with UV light. This is because the dye contains fluorescent molecules that absorb light of a certain wavelength (such as UV light) and re-emit it at a longer wavelength, such as yellow, orange or pink in the visible spectrum. This isn’t particularly noticeable in daylight, but is more obvious under UV light.
References
Web References
- w1 – If you wish to purchase reinforced plastic lids for the Tic Tac® boxes, you can contact Bernd Mößner (info@experimente-zur-energiewende.de) for more information and to place an order.
Resources
- For more information about fingerprint analysis, visit the Forensic Science Simplified website.
Review
The article describes a simple method that utilises easily sourced equipment to illustrate a contemporary method for visualising latent fingerprints. Most suitable for 16–19-year-old chemists, the technique allows students to consider an alternative take on polymerisation as a valuable tool in forensic detection, rather than focusing on its role in the manufacturing of materials.
I trialled the method with two classes of students (aged 16–17) who commented on how much they enjoyed that the method used common, everyday equipment to illustrate an interesting scientific technique. The method is easy to follow, gives good results and is reproducible.
This activity is definitely going to be incorporated in my chemistry teaching, as it is a novel task, will allow me to provide my students with the stretch and challenge of considering an anionic mechanism for polymerisation, and will encourage discussion around science careers in forensics and criminology.
Caryn Harward, head of chemistry, St Mary’s Calne, UK





