Chewing flavours Teach article
Ken Gadd and Luca Szalay introduce a procedure used in industry – and adapted for school students – to measure the citric acid level in chewing gum.
How acidic is chewing gum?

How many people do you know who chew gum? Why do they? You could try a straw poll, but the most likely answer will be for the flavour. Chewing gum consists of a water-insoluble gum base and water-soluble ingredients such as flavourings and sweeteners. When you chew it, the flavourings and sweeteners are released, dissolved and swallowed (hopefully the gum stays in the mouth). Once the flavour has been extracted, the gum is discarded. Unfortunately it sticks strongly to hard surfaces such as concrete and is very hard to remove.
There’s plenty of evidence for this if you look at the pavements you walk on. But back to flavouring….
A common ingredient in chewing gum and other confectionary is citric acid. It gives that sharp, refreshing citrus taste that ‘attacks’ the tongue. The packaging will tell you how much citric acid is in the chewing gum. It’s the role of an analytical chemist in the quality control laboratory to check it out. The method below is based on the standard procedure used by a company that manufactures various flavours of chewing gum and bubble gum (a type of chewing gum). It has been adapted for use in schools and colleges so that your students can mimic authentic workplace practice.
Here are some questions that could be used to get students thinking:
- How many people do you know who chew gum?
- How old are they?
- What makes bubble gum different from other types of chewing gum?
- How do you remove chewing gum from clothing?
- Why do sugar-free gums still taste sweet?
- How could you work out how much acid is in chewing gum?
The StandardBase project
The StandardBase project has produced 72 standard analytical procedures, used in industry and adapted for use by students in schools and colleges, accessible via the Internet. For more information about the project, see ‘StandardBase: a Leonardo da Vinci pilot project for practical education and training in chemistry’ in this issue of Science in School.
The procedure: Determination of citric acid in Hubba Bubba® chewing gum using acid-base titration
| Duration | 1 hour |
|---|---|
| Analytical instruments used | Volumetric glassware |
| Sample specification | Orange flavoured Hubba Bubba® bubble gum |
| Supplied by | Most confectionary shops and supermarkets |
| Component to be determined | citric acid |
| Method(s) | titrimetry acid-base |
| Field(s) | food and beverages |
Before they start the practical, teachers may want their students to understand the chemistry of the analysis. To check they have an appropriate knowledge, students might try the interactive test on the StandardBase websitew1. To help their work, students can also download a file about titrationw2.
Scope
The aim of this analytical procedure is to determine the citric acid content in Hubba Bubba® bubble gum. This bubble gum is available in the UK and most parts of Europe. It is manufactured by Wrigleyw3 in Plymouth, UK. The method described here is based on an analytical procedure used by Wrigley in their Plymouth laboratories.
Principle
The determination is based on an acid/base reaction between the citric acid in the bubble gum and standard sodium hydroxide. The citric acid content of the bubble gum can be calculated from titration results.
Equipment
- Kitchen pastry roller (rolling pin)
- 250 cm3 conical flask
- 250 cm3 graduated flask
- 100 cm3 graduated flask
- Magnetic stirrer and follower (also known as a flea)
- 10 cm3 burette (reading to nearest 0.02 cm3)
- Top pan analytical balance
- Wood block
- Knife / scissors
Materials and their CAS numbersw4
- Orange flavoured Hubba Bubba®
- Sodium hydroxide (CAS number: 1310-73-2)
- Phenolphthalein (CAS number: 77-09-8)
Reagent solutions
- Standard 0.10 mol dm-3 sodium hydroxide. If this is not available, dissolve 1.00 g of sodium hydroxide in about 100 cm3 of pure water. Wash carefully to a 250 cm3 graduated flask and make up to the graduation mark. Homogenise the solution. Standardise by titration with 0.10 mol dm-3 dhydrochloric acid, itself standardised against solid potassium hydrogencarbonate.
- Phenolphthalein indicator. Weigh out 0.20 g of phenolphthalein and dissolve in about 50 cm3 of methanol. Transfer solution to a 100 cm3 graduated flask, make up to the graduation mark with methanol and homogenise the solution.
Procedure
- Take one orange flavoured Hubba Bubba® bubble gum piece, unwrap it and place onto a wood block.
- With the rolling pin, roll the bubble gum into a very thin strip approximately 160 x 30 x 0.5 mm.
- Cut the thin strip into small pieces about the size of long grain of rice.
- Weigh out 1.00 g of orange flavour Hubba Bubba® bubble gum bits.
- Add to 100 cm3 of pure water contained in a 250 cm3 conical flask. Add a magnetic follower and stopper.
- Stir vigorously for 30 min, making sure bubble gum bits do not lump together.
- Add 0.50 cm3 of phenolphthalein indicator and titrate with 0.1 mol dm-3sodium hydroxide contained in a 10 cm3 burette. The end point is pink.
- Repeat twice more and average the three results. Average titration = V cm3.
Calculations
Use the following to calculate the percentage by mass of citric acid monohydrate in the Hubba Bubba® bubble gum:
- Each cubic centimetre of 0.10 mol dm-3 sodium hydroxide is equivalent to 7.00 mg of citric acid monohydrate.
- % by mass citric acid monohydrate = 0.70V
Note: a correction factor is necessary if the sodium hydroxide solution is not exactly 0.10 mol dm-3.
Expression of results
Give the mass of citric acid monohydrate in Hubba Bubba® bubble gum in percentage by mass (mass of citric acid monohydrate in 100 g of bubble gum). The manufacturer’s allowed range is 1.9–2.1 percentage by mass.
Precision
The precision of the analysis is determined by the burette readings. An inexperienced student might read a burette to a precision of ± 0.05 cm3. More experienced students might read to a ± 0.02 cm3.
The graph
The graph below shows the results obtained by students in other European countries (accurate at time of going to press). If your students are interested in finding out the latest status, they can view the most up-to-date results online.
If your students want to add their own results to the graph, email Luca Szalay (luca@chem.elte.hu). You will get a username and password to be able to use this function of the StandardBase database. Then you can register your students, which will allow them to upload their numbers on the graph.
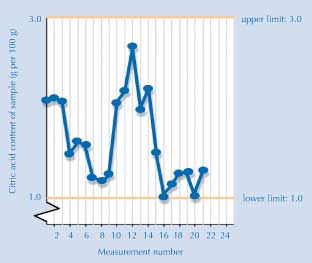
Image courtesy of Luca Szalay
Web References
- w1 – Teachers may like to get their students to try the interactive test on the StandardBase website: www.vapro-ovp.com/applications/toetsing/vraag.asp?toetsID=29
- w2 – A file about titration can be downloaded from the StandardBase website: www.standardbase.com/tech/uk-voltech.pdf
- w3 – For more information about Hubba Bubba® products see www.wrigley.co.uk/index.cfm?articleid=138
- w4 – The CAS Registry is the largest and most current database of chemical substance information in the world. See www.cas.org/expertise/cascontent/registry/regsys.html
Review
This article offers an interesting and original approach to acid-base reactions and acid-base titrimetry, two topics usually regarded as heavy and boring (remember when you were a student?).
Starting from a familiar object (chewing gum), with its characteristics and practical problems, the authors present the analytical procedure (determination of citric acid in Hubba Bubba® chewing gum), widening the subject to theoretical aspects with the help of web references. Chemistry teachers could use the article to discuss the importance of standard analytical procedures, in addition to the topics of acid-base reactions and acid-base titrimetry. The proposed analysis gives the opportunity for the mathematical treatment and sharing of the obtained data, thus linking chemistry, statistics and the English language.
The link to a real job in a quality control laboratory, the richness of didactic materials in the StandardBase website (preliminary tests, theory, graphs and protocols) make ‘Chewing flavours’ a useful resource for chemistry teachers and students. It could be used to discuss the role of the analytical chemist in the context of industry and research and / or to prepare a visit to a factory or to a laboratory.
The article could be useful in upper secondary or vocational schools in Italy not only to test the proposed activity but also the other procedures of StandardBase project that are available on the website. The possibility to join the project is another interesting feature of the article.
Teachers may like to pose the following comprehension questions:
The substance that gives orange Hubba Bubba® its flavour is:
a) malic acid
b) citric acid
c) ascorbic acid
d) hydrochloric acid
The concentration of the titrating solution used in the procedure is:
a) 0.10 g dm-3
b) 1.00 % by mass
c) 0.10 mol dm-3
d) 1.00 mol dm-3
Following the authors’ suggestion, I would find a sequel on the chemical aspects of removing chewing gum from surfaces and clothes interesting.
Giulia Realdon, Italy





