School experiments at the nanoscale Teach article
Eleanor Hayes highlights some education resources about the nanoscale and nanotechnology.
iStockphoto
With the help of many education projects, introducing the nanoscale at school has never been easier – whatever the age of your students. Below are two experiments (for children aged 8+ and for 14- to 16-year-olds); many more resources are listed at the end of the article.
Dilution and the sense of smell

Deutsches Museum
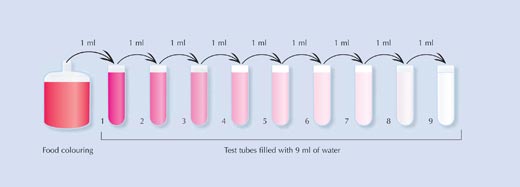
In the following experiment, suitable for ages 8 and above, food colouring is serially diluted, causing the colour and smell to fade gradually. The colour will fade more quickly that the smell, illustrating that even though our eyes cannot detect the chemical responsible for the colour, it is still present, as verified by the smell.
In the same way as we use our eyes to see large things and our nose to smell small things, nanoscientists use special tools to analyse (and manipulate) things at the very small scale: the nanoscale. Atomic force microscopes can feel and move individual atoms, while special surfaces with nanotextures on them can repel water extremely efficiently.
The experiment is taken from the ‘Time for nano’ project, which offers informal education materials about the benefits and risks of nanoscale research, engineering and technology. The website and materials are available in nine languages (Dutch, English, Finnish, French, German, Italian, Polish, Portuguese and Turkish). The project members – science centres across Europe – also offer ‘nanodays’, with demonstrations, experiments, games, meetings and discussions about nanotechnology. For more information, see the Time for Nano websitew1.
Introduction
When introducing the activity, the following examples may help to illustrate how small the nanoscale is.
- Our nails grow 1 nm each second.
- The virus most usually responsible for the common cold has a diameter of 30 nm.
- A cell membrane is around 9 nm across.
- The DNA double helix is 2 nm across.
- The diameter of one hydrogen atom is around 0.2 nm.
Encourage students to consider the things that they cannot see directly, for example the ozone layer, dyes in stained glass windows or the colloidal nature of milk.
Explain that the olfactory bulb of the brain is responsible for interpreting the smells that the nose detects. The olfactory bulb is strongly linked to a part of the brain that is responsible for remembering things, which is why certain smells can make us remember specific things clearly.
The students can calculate that in each tube, the food colouring is ten times more dilute than the previous tube. By the time they reach Tube 9, the original food colouring has been diluted to the level of one part of food colouring to a billion parts of water.
Materials
- Some scented (this is important) food colouring
- A Pasteur pipette
- Nine test tubes, numbered 1-9.
Procedure
Deutsches Museum
- Carefully fill each test tube with 9 ml of water.
- Using the Pasteur pipette, carefully add 1 ml of food colouring to Tube 1. Mix the tube thoroughly.
- Smell the contents. What does it smell of? Does it smell the same as the original food colouring?
- Now take 1 ml of liquid from Tube 1, add it to Tube 2 and mix thoroughly.
- Continue the process by repeating steps 3 and 4: dilute Tube 2 into Tube 3, Tube 3 into Tube 4, and so on.
At what point can you no longer see any colour in the tubes?
At what point can you no longer smell anything in the tubes?
How can you explain the difference?
The method you have just used is called a serial dilution. If you wanted, in just one step, to dilute 1 ml of the food dye to the same concentration as in Tube 9, how much water would you need?
Safety notes
- Do not eat the food colouring.
- Some people might be intolerant of the food dye. If it comes into contact with skin, wash it off with lots of water.
- Avoid getting the food colouring on clothes, as it will stain.
Building a liquid crystal thermometer
Liquid crystals have properties between those of a conventional liquid and those of a solid crystal; for example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a particular direction, as in a crystal. Liquid crystals are sensitive to external factors, such as temperature, and change their molecular arrangement when these factors vary. In response to a change in temperature, some types of liquid crystal (thermotropic ones) change colour as a result of a change in assembly.

iStockphoto
In this experiment, students investigate colour changes in a thermotropic liquid crystal and then build their own liquid crystal thermometer. The protocol, which is suitable for students aged 14-18, was developed as part of the ‘Nanoyou’ project. Extensive supporting materials are available (in Danish, English, German, Italian, Portuguese and Slovak) from the project websitew2.
The Nanoyou website is available in 12 languages and offers a range of free materials, including posters, presentations, card games, role plays and a teachers’ training kit. This kit covers the fundamental concepts in nanoscience and nanotechnologies; applications of nanotechnologies; four laboratory experiments and a virtual experiment. Separate kits are available for the 11-13 and 14-18 age groups.
Schools that participate in the project work directly with leading European nanoscience research centres, receiving nanotech-related materials and taking part in workshops. Particular focus is given to ethical, safety and social implications, as well as present and future limits to scientific development. To learn how to get involved, visit the Nanoyou websitew2.
Preparation
For this experiment, you will need four different mixtures prepared from three liquid crystals:
- Cholesteryl oleyl carbonate (CAS number 17110-51-9, Sigma-Aldrich 151157; 25 g costs about €60)
- Cholesteryl pelargonate (CAS number 1182-66-7, Sigma-Aldrich C78801; 100 g costs about €115)
- Cholesteryl benzoate (CAS number 604-32-0, Sigma-Aldrich C75802; 25g costs about €40)
The instructions for preparing each of these liquid crystal mixtures (‘Student synthesis procedure’) and supporting material for teachers are available to download from the Nanoyou websitew2.
Materials
- 4 pre-prepared liquid crystal mixtures
- 4 10-ml vials
- A sheet of sticky-back plastic (used e.g. for covering books)
- A spatula
- Scissors
- A permanent marker pen
- A room thermometer
- An A4 sheet of white paper
- An A4 sheet of black paper
- A water bath (or a hotplate, a Pyrex glass water container half filled with water, and thermometer)
- A clothes peg
- An A4 sheet of foam
- An A4 sheet of black card
- Gloves
- Safety glasses
- Paper tissue
- Sticky tape (optional)
- A hairdryer (optional)
Safety notes
Solids should not be inhaled. Wear gloves and safety glasses; contact with skin, eyes or clothing should be avoided. Wash your hands thoroughly after handling the liquid crystals.
Procedure
Preparing the liquid crystal sheets
- Using Table 1, prepare four different liquid crystal mixtures, each of which is sensitive to different ranges of temperatures.
| Liquid crystal mixture | Sensitivity temperature (°C) | Cholesteryl oleyl carbonate | Cholesteryl pelargonate | Cholesteryl benzoate |
|---|---|---|---|---|
| Mixture 1 | 17–23 | 0.65 | 0.25 | 0.10 |
| Mixture 2 | 26.5–30.5 | 0.45 | 0.45 | 0.10 |
| Mixture 3 | 32–35 | 0.40 | 0.50 | 0.10 |
| Mixture 4 | 37–40 | 0.30 | 0.60 | 0.10 |

sheet
Image courtesy of the
‘Nanoyou’ project
- Cut two pieces of sticky-back plastic (about 10 x 10 cm), peel off the backing paper and lay the plastic (sticky side up) on the bench.
- Place 2-3 spatulas of the liquid crystal mixture 1 in the centre of one of the sheets. If the liquid crystal has solidified, warm the vial first with a hairdryer until the mixture has the consistency of honey.
- Place the second piece of sticky-back plastic on top of the first piece, sticky side down. As you do this, gently press the middle to distribute the liquid crystal evenly, creating a thin layer of liquid crystal about 4 x 4 cm. Do not press too hard, otherwise the mixture will leak out; if it does, clean it up immediately with paper tissue.
- Cut off any excess plastic and label the corner of the sheet with the number of the liquid crystal mixture (1).
- Repeat the same procedure for liquid crystal mixtures 2-4.
Use the room thermometer to determine the temperature in the room you are in.
Could any of your liquid crystal mixtures be used to determine the room temperature? If so, which one?
Investigating the temperature changes
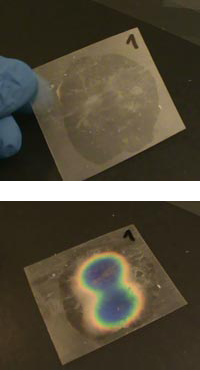
before and after it has been
touched
Images courtesy of the
‘Nanoyou’ project
- Place your four liquid crystal sheets on a sheet of white paper. Wait few seconds. What do you see?
- Wearing gloves, press your finger against each of the liquid crystal sheets. (To make a fair comparison, you need to keep your finger on each liquid crystal sheet for the same length of time.) What do you see now?
- Repeat the procedure on a sheet of black paper.
- Record your observations in Table 2. (Tables 2-6 can be downloaded from the Science in School websitew3.)
Why do you get different results when you test the liquid crystal sheets against white paper and black paper?
Did all four liquid crystal sheets display some colour? If not, why not? What could you do to make these sheets display colour?
- Rub your hands together and test each of the sheets again. Do you see any difference?
| White paper | Black paper | |
|---|---|---|
| Sheet 1 (mixture 1) | ||
| Sheet 2 (mixture 2) | ||
| Sheet 3 (mixture 3) | ||
| Sheet 4 (mixture 4) |
Testing the liquid crystal sheets

sheet in the water bath
Image courtesy of the
‘Nanoyou’ project
- Fill the water bath with cold water and raise the temperature to 15 °C.
- Keep a thermometer in the water to check the temperature.
- Place a sheet of black paper behind the water bath, to make any colours visible.
Note: the paper should not touch the hotplate. - Holding it with a clothes peg, immerse liquid crystal sheet 1 in the water bath (left). Can you see any colour?
- Raise the water temperature to 23 °C. In Table 3, record the colours that you see as the temperature increases.
| Liquid crystal sheet number 1 | ||
|---|---|---|
| Temperature (°C) | Colour | Comments |
| 16 | ||
| 17 | ||
| 18 | ||
| 19 | ||
| 20 | ||
| 21 | ||
| 22 | ||
| 23 | ||
At what temperature do you start to see some colour in sheet 1? Does this correspond to the temperature predicted in Table 1?
Does the order of colours that you have recorded in the table above follow a particular pattern? If so, what pattern and why do you think this might be?
- Take the liquid crystal sheet out of the water bath. Does it lose its colour immediately? If not, why not?
Imagine putting sheet 1 in a water bath of unknown temperature. If the sheet turned orange what temperature would the water be?
- Place liquid crystal sheet 2 in the water bath (now at 23 °C) and raise the temperature to 30 °C. Record your observations in Table 4.
| Liquid crystal sheet number 2 | ||
|---|---|---|
| Temperature (°C) | Colour | Comments |
| 22-23 | ||
| 23-24 | ||
| 25 | ||
| 26 | ||
| 27 | ||
| 28 | ||
| 29 | ||
| 30 | ||
- When the water temperature reaches 30 °C, test sheet 1 again. Can sheet 1 detect temperatures around 30 °C? Why/why not?
- Place liquid crystal sheet 3 in the water bath (now at 30 °C) and raise the temperature to 35 °C. Record your observations in Table 5.
| Liquid crystal sheet number 3 | ||
|---|---|---|
| Temperature (°C) | Colour | Comments |
| 30 | ||
| 31 | ||
| 32 | ||
| 33 | ||
| 34 | ||
| 35 | ||
- Place liquid crystal sheet 4 in the water bath (now at 35 °C) and raise the temperature to 40 °C. Record your observations in Table 6.
| Liquid crystal sheet number 4 | ||
|---|---|---|
| Temperature (°C) | Colour | Comments |
| 35 | ||
| 36 | ||
| 37 | ||
| 38 | ||
| 39 | ||
| 40 | ||
Was the colour sequence that you observed for sheets 2-4 the same as for sheet 1? Why/Why not?
When you take sheets 2, 3 and 4 out of the water bath, do they behave like sheet 1? If not, what is the difference? Why?
Which of the four liquid crystal mixtures would you use to see whether you have a raised temperature? Why?
Constructing the liquid crystal room thermometer
- On the sheet of white foam, use the permanent marker pen to write the word ‘NANO’. You will need to cover each letter with one of the four liquid crystal sheets, so make sure the single letters are large enough but not too large (see image below).
- Cut the four letters out of the foam, leaving a single sheet of foam with holes spelling the word ‘nano’.
- Turn the sheet of foam over and cover each letter with one liquid crystal sheet, as follows:

The finished thermometer
Image courtesy of the
‘Nanoyou’ projectN – sheet 1
A – sheet 2
N – sheet 3
O – sheet 4 - Using sticky-back plastic or sticky tape, fasten the liquid crystal sheets to the foam, making sure that each letter only exposes one sheet.
- Cover the liquid crystal sheets with the sheet of black card, fastening it to the white foam. Your room thermometer is now complete.
Does your room thermometer show any colour? If not, why not?
If your thermometer does not show any colour, try placing it over a working laptop computer. It will demonstrate what we all know – that they heat up.
Your thermometer will last 3-6 months, after which it can be disposed of as normal waste.
How is this nano?
The properties of materials at the macroscale are affected by the structure of the material at the nanoscale. Changes in a material’s molecular structures are often too small to see directly with our eyes, but sometimes we can see changes in the material’s properties. Liquid crystals are an excellent example, in particular the type used in this experiment, since their optical properties (colour) change visibly as the temperature of the liquid crystal is changed. In nanotechnology, scientists take advantage of the peculiar properties of materials at the nanoscale to engineer new materials and devices.
Acknowledgements
Time for Nano is funded by the European Commission under the 7th Framework Programme.
The liquid crystal experiment published on the Nanoyou website was adapted from the ‘Preparation of cholesteryl ester liquid crystals’ – one of the many experiments listed on the website of the University of Wisconsin-Madison, USAw4 – and from the ‘Exploring materials: liquid crystals’ activity developed by the Nanoscale Informal Science Education networkw5.
The ‘Nanoyou’ (Nano for Youth) project is funded by the European Commission under the 7th Framework Programme (FP7/2007-2013) under grant agreement 233433.
Web References
- w1 – To learn more about the Time for Nano project, enter the video competition, download experimental protocols or find out about forthcoming events, see: www.timefornano.eu
- w2 – Supporting materials for the liquid crystal experiment, including details of how to synthesise the liquid crystals, are available from the Nanoyou project website (www.nanoyou.eu) or via the direct link: http://tinyurl.com/2ulmsta
- w3 – All the tables needed for recording the results of the experiment can be downloaded from the Science in School website: www.scienceinschool.org/2010/issue17/nano#resources
- w4 – For the instructions for preparing cholesteryl ester liquid crystals, see the website of the University of Wisconsin-Madison’s Materials Research Science and Engineering Center (http://mrsec.wisc.edu) or use the direct link: http://tinyurl.com/34kq8qn
- w5 – For the ‘Exploring materials: liquid crystals’ activity, see the website of the Nanoscale Informal Science Education network (www.nisenet.org) or use the direct link: http://tinyurl.com/35el37p
Resources
- For some nanotechnology experiments published in a previous issue of Science in School, see:
- Mallmann M (2008) Nanotechnology in school. Science in School 10: 70-75. www.scienceinschool.org/2008/issue10/nanotechnology
- The Nano and Me website includes a discussion of what we mean by ‘nano’ in food. See: www.nanoandme.org/nano-products/food-and-drink
- To learn more about our sense of smell, see the Nanooze website: www.nanooze.org/english/articles/5senses_noseknows.html
- For a video of serial dilution, see the website ‘Dr Shawn’s Science Fair Success Series’: http://web.mac.com/drshawn1
- The Nano mission website offers downloadable educational games that introduce basic concepts in nanoscience through real-world practical applications, from microelectronics to drug delivery. See: www.nanomission.org
- The Wellcome Trust’s free Big Picture series for teachers and students (aged 16 and above) explores issues around biology and medicine. It can be downloaded or ordered online.
- In June 2005, the Big Picture focused on nanotechnology. See: www.wellcome.ac.uk or use the direct URL: http://tinyurl.com/344mpws
- The Discover Nano website from the Northwestern University, Chicago, USA, offers an interactive timeline tracing the history of nanotechnology from medieval glass to the present. See: www.discovernano.northwestern.edu
- The Understanding Nanotechnology website offers an introduction to nanotechnology; explanations of nanotechnology applications, including medicine, fuel cells and food; and a discussion of nanomaterials. See: www.understandingnano.com
- For German speakers, Science on Stage Germany has produced a 120-page textbook describing the complexity and diversity of the nano-world, covering research, applications, history, education and job offers. Teaching tips, teaching materials and worksheets are also available. The book costs €2.50. For more information, see: www.scienceonstage.de or use the direct link: http://tinyurl.com/3yqgasa





