Elements in focus: molybdenum Understand article
From samurai swords to healthy tomato plants, this little-known element has wider uses than you might expect.

from the Climax mine in
Colorado, USA
James St. John / Flickr
Only around 90 chemical elements occur in nature. Some of these are very familiar – from lustrous precious metals such as gold and platinum, to the oxygen in the air we breathe and the carbon that forms the backbone of the molecules of life. Many other elements are much less familiar – but, as members of this select band of 90, perhaps even the more obscure elements deserve to be slightly better known.
In this article, we push one of these low-celebrity elements – molybdenum – into the spotlight and reveal its unique history and character.
What is molybdenum?
To look at, molybdenum is unremarkable: a hard, silvery-grey metal, with a density some 30% more than iron. In nature, it occurs only as a compound – mostly in the form of the mineral molybdenite, MoS2. And although molybdenum has been known since ancient times, the story of its identification began with confusion.
The word ‘molybdenum’ comes from the ancient Greek word for lead, molybdos. Like lead and graphite, molybdenite can be used to make a mark on a surface, so for centuries it was thought to be just another lead-containing mineral. In the late 18th century, during the early decades of modern chemistry, some people began to suspect that molybdenite was in fact a different substance from lead or graphite. In 1778, the great Swedish chemist, Carl Wilhelm Scheele – who also played a major role in the discovery of oxygen and several other chemical elements – proved chemically that molybdenite was in fact a sulfur compound of a new, unidentified element. Another Swedish chemist, Peter Jacob Hjelm, then isolated the metal in 1781, but it was not properly purified until several decades later.
On Earth, molybdenum is not abundant: it ranks 53rd among elements in Earth’s crust. Pure, elemental molybdenum has never been found on Earth (only as a compound with other elements) – but the Russian Luna 24 mission to the Moon in 1976 brought back a tiny piece of pure molybdenum. Even in space, molybdenum is rare, as – unlike lighter metallic elements – it is not made in normal nuclear fusion processes in stars, but is created in supernova explosions.

Nicole Kinsman / IMOA
Molybdenum facts
- Element name: molybdenum
- Atomic number: 42
- Relative atomic mass: 95.96
- Density: 10.22 g/cm3
- Melting point: 2623 °C
- Coefficient of thermal expansion: 4.8 x 10-6 / K at 25 °C
- Valency: oxidation states from –II to VI
Special characteristics
Molybdenum’s most remarkable physical characteristics are its very high melting point, over 1000 °C higher than that of iron, and its very low expansion when heated. This means molybdenum is a good material to use where stability at high temperatures is needed, such as in furnaces. A common use until recently was in glowing filament light bulbs, where pure molybdenum wire was used to support the hot filament.
Chemically, molybdenum is closely related chemically to tungsten, which is situated directly below it in the periodic table, and it shares with tungsten the ability to form very hard alloys with iron and other elements. Molybdenum is a transition metal, and so – like other transition elements – it can form compounds with varying valency (the number of electrons used in bonding). This is because the atoms in transition metals have spaces not only in their outermost electron shell but also in a lower shell, because of the shells’ overlapping energy levels. This rather fluid arrangement is the basis for molybdenum’s role in living organisms as an electron carrier, because it can loan and accept a variable number of electrons at the same time.

Antoine2K / Shutterstock.com
Vital for life
Molybdenum is vital for life, in both plants and animals. It is the only essential trace element within the second row of the transition elements in the periodic table, which runs from yttrium (atomic number 39) to cadmium (atomic number 48). Molybdenum-containing enzymes are found in both bacteria and archaea, two of the most ancient forms of living organisms. Based on this finding, some scientists speculate that molybdenum may have been present in the earliest life forms on Earth – and also in the ‘last universal common ancestor’ of all living things.
Whatever the case, it’s certain that molybdenum-containing enzymes are essential to plants. Some plants can obtain the nitrogen they need through a symbiotic relationship with nitrogen-fixing bacteria in their roots. These bacteria use nitrogenase enzymes containing molybdenum to capture atmospheric nitrogen and turn it into useful nitrogen compounds (Hernandez, 2009), including amino acids and proteins. Plants can also use nitrogen compounds (such as nitrates) in the soil with the help of another molybdenum enzyme, nitrate reductase. A study carried out in 1939 with tomato plants first established molybdenum as an essential nutrient in plants (Arnon & Stout, 1939).
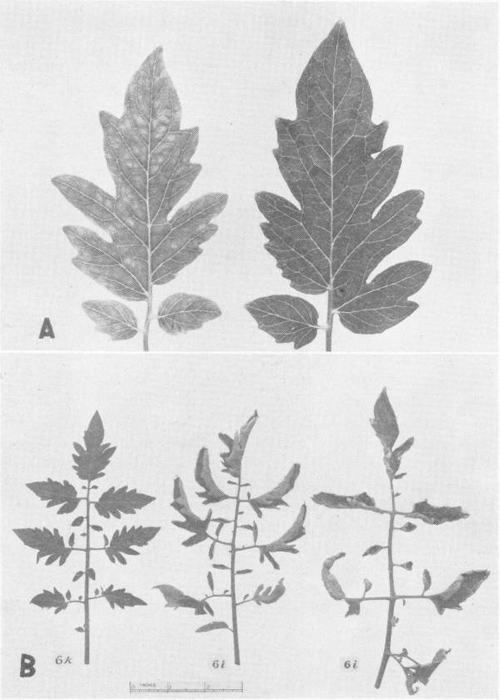
American Society of Plant Biologists, reprinted with permission
Many animals require molybdenum too: the average human needs to take in around 45 micrograms of molybdenum daily, which we normally obtain from vegetables (1 kg of dehydrated vegetables contains around 1 milligram of molybdenum), as well as from milk and nuts. The metal is a component of several metabolic enzymes, including sulfite oxidase. This enzyme breaks down the sulfite formed from amino acids and thus prevents sulfite accumulating in the body, where it can cause fatal damage. Another important molybdenum enzyme is xanthine oxidase, which breaks down xanthine (found in meat and other foods) to uric acid; this is then removed from the body by the kidneys. For this reason, xanthine oxidase inhibitors are used to treat gout – a disease in which excess uric acid builds up in the joints and muscle tendons, causing pain.

Susan Watt
Technology and industry
A remarkably early technological use of molybdenum dates from 14th-century Japan: a samurai sword from this era has been found to contain molybdenum, which would have helped to enhance the legendary strength and sharpness of such blades.
In the West, molybdenum’s first industrial use came in the late 19th century as a substitute for tungsten in steel-making. By the time of World War I, tungsten supplies were so depleted that molybdenum started being mined on a large scale, particularly in the USA. The main source was the mine at Climax, Colorado, USA. Starting in 1915, for many years this mine supplied three-quarters of the world’s molybdenum.

Gord McKenna / Flickr
Molybdenum was used in Germany during World War I to make steel for military hardware – gun barrels, armour plating, shells and submarine parts. Adding a small amount (1–2%) of molybdenum dramatically improved the steel’s strength: shells that had easily penetrated 75 mm armour plating became powerless against a 25 mm molybdenum steel plate. This ‘German steel secret’ was followed by the development of molybdenum steel in other countries.
Molybdenum today
Today, the main use for molybdenum is still in alloys, particularly steel. One much-used alloy (316L stainless steel) contains 2–3% molybdenum and is tough, corrosion resistant and hypoallergenic, so it is used for a huge range of products, from smartphone cases to body-piercing jewellery, as well as in building construction.
Molybdenum compounds are also used in high-tech electronics. Here, they help to provide the touch-screen technology increasingly used in smartphones and tablets, as well as being used in more conventional liquid-crystal displays (LCDs) and solar panels.
Molybdenum is also a component of many ‘permalloy’ materials used in electronic devices, with uses ranging from power supplies and transformers to microelectronic components. Space agencies NASA and ESA have used molypermalloy (MPP) materials in missions to Jupiter, Saturn and Mars. The Cassini-Huygens mission used these materials in its on-board mass spectrometer, which sent back data on the atmosphere of Saturn and its moons. So molybdenum may one day help us to detect life on another planet – as well as sustaining life on our own.
ESA
Acknowledgments
The authors would like to thank Dr Ulrike Kappler for her advice on the article.
References
- Arnon DI, Stout PR (1939) Molybdenum as an essential element for higher plants. Plant Physiology 14(3): 599-602. doi: 10.1104/pp.14.3.599
- Hernandez JA, George SJ, Rubio LM (2009) Molybdenum trafficking for nitrogen fixation. Biochemistry 48(41): 9711-9721. doi: 10.1021/bi901217p
Resources
- For a comprehensive account of molybdenum at an introductory level, see:
- Lepora N (2007) The Elements: Molybdenum. New York, USA; Marshall Cavendish. ISBN: 0761422013
- Find out more about the chemical element molybdenum and its place in the periodic table.
- The website of the International Molybdenum Association has plenty of useful resources on this element, its role in biology and its applications.
- For information on the uses of molybdenum in products and industry, see the website of HC Starck.
- Find out more about how heavy elements are made in stars:
- Rebusco P, Boffin H, Pierce-Price D (2007) Fusion in the Universe: where your jewellery comes from. Science in School 4: 52-56.
Review
This article could be used to deepen students’ knowledge about molybdenum, one of the less well known elements that occur in nature. It can also be a starting point for discussing the essential role of such little-known elements in life, and to raise awareness of the importance of research in the development of technology.
Possible comprehension questions include:
- Can you name two scientists involved in the discovery of molybdenum? Explain their role in the discovery of this element.
- Why is molybdenum a good material to use where stability at high temperatures is needed?
- Why are molybdenum enzymes essential to plants?
- Why was molybdenum used in samurai swords?
- Can you mention three everyday products that contain molybdenum?
Mireia Güell Serra, chemistry teacher, INS Cassà de la Selva, Spain





